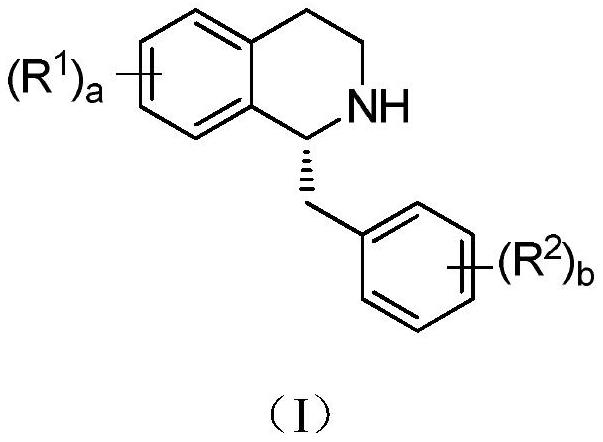
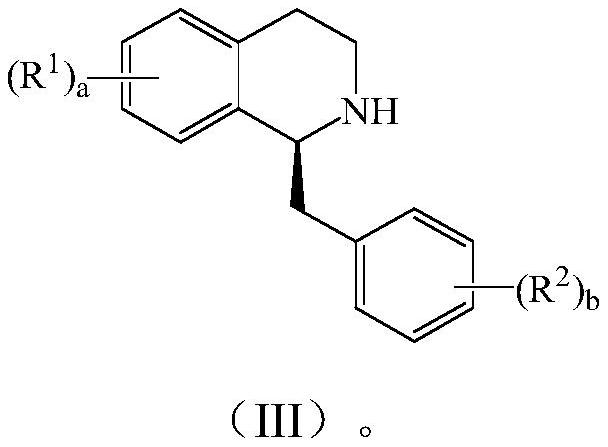
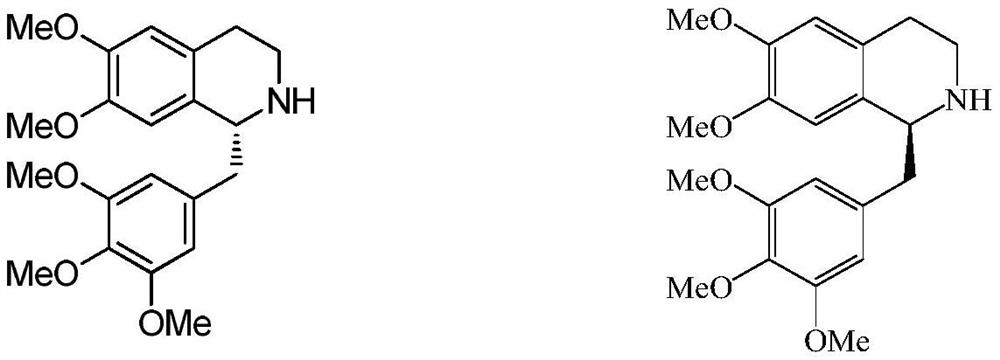Preparation method of tetrahydrobenzyl isoquinoline compound
A compound and mixture technology, which is applied in the field of preparation of tetrahydrobenzylisoquinoline compounds, can solve the problems affecting the total yield and quality of mivacurium chloride finished products, many impurities in the product, low chemical purity and optical purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Weigh 2.6 g of catalyst (S,S)-N-(p-toluenesulfonyl)-1,2-diphenylethanediamine (p-cumene) ruthenium (II) chloride, add 100 mL A mixture of dichloromethane and 400 mL of formic acid / triethylamine (5:2) was prepared as a standby solution. Weigh 150.0 g of 6,7-dimethoxy-1-(3,4,5-trimethoxybenzyl)-3,4-dihydroisoquinoline, dissolve it in 200 mL of dichloromethane, and add the above-mentioned standby solution , the reaction was complete at room temperature. Aqueous sodium hydroxide solution was added to the reaction solution to quench the reaction, the layers were allowed to stand, and the organic phase was concentrated.
[0068] Add ethanol to dissolve, then add 55 g of D-tartaric acid, and reflux under stirring until the reaction is complete. Stir to cool and crystallize, and filter. The filter cake was dissolved in water, stirred with activated carbon, and filtered. The filtrate was added with aqueous sodium hydroxide solution to adjust pH to 10, extracted wi...
Embodiment 2
[0069] Example 2: Weigh 1.3 g of catalyst (S, S)-N-(p-toluenesulfonyl)-1,2-diphenylethanediamine (p-cumene) ruthenium (II) chloride, add 300 mL of N , N-dimethylformamide and 150 mL of formic acid / triethylamine (5:2) mixed solution to prepare a standby solution. Weigh 150.0g of 6,7-dimethoxy-1-(3,4,5-trimethoxybenzyl)-3,4-dihydroisoquinoline and dissolve it in 300mL of N,N-dimethylformamide , add the above-mentioned stock solution, and react at room temperature until the reaction is complete. Aqueous potassium hydroxide solution was added to the reaction solution to quench the reaction, extracted with ethyl acetate, the ethyl acetate layer was washed with water and saturated brine, respectively, the layers were separated, and the organic phase was concentrated.
[0070] Add methanol to dissolve, then add 50 g of D-malic acid, and reflux with stirring until the reaction is complete. Stir to cool and crystallize, and filter. The filter cake was dissolved in water, stirred wit...
Embodiment 3
[0071] Example 3: Weigh 20g catalyst (S,S)-N-(p-toluenesulfonyl)-1,2-diphenylethanediamine (p-cumene) ruthenium(II) chloride, add 3000mL acetonitrile and 4500mL formic acid / triethylamine (5:2) mixed solution to prepare a standby solution. Weigh 1500g of 6,7-dimethoxy-1-(3,4,5-trimethoxybenzyl)-3,4-dihydroisoquinoline, dissolve it in 3000mL of acetonitrile, add the above-mentioned stock solution, and at room temperature React until the reaction is complete. The reaction solution was concentrated, an aqueous sodium hydroxide solution was added to quench the reaction, extracted with dichloromethane, the dichloromethane layer was washed with water and saturated brine respectively, the layers were separated, and the organic phase was concentrated to dryness.
[0072] Add isopropanol to dissolve, then add 600 g of D-mandelic acid, and reflux under stirring until the reaction is complete. Stir to cool and crystallize, and filter. The filter cake was dissolved in water, stirred wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com