Cisatracurium besylate with new crystal form and purifying method thereof
Atracurium cissulfonate and a purification method are applied in the field of new crystal form atracurium cissulfonate and its purification, which can solve the problems of difficulty in reproduction and the like, and achieve good stability and purification method. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A new crystal form of atracurium cis-benzenesulfonate and its purification method, comprising the following steps:
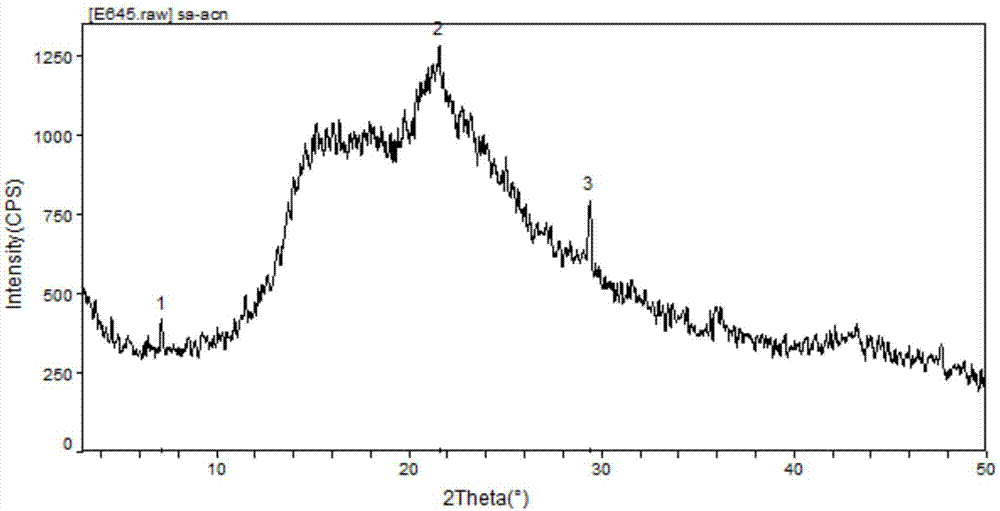
[0034] (1) Preparation of amorphous atracurium cis-benzenesulfonate: Add 700mL of acetonitrile to a 2L reaction flask, stir, add 140g of crude atracurium cis-benzenesulfonate at 10±2°C, stir for 30-60min; filter , collect the filtrate; slowly add the filtrate into a 5L reaction flask containing 2800mL ether, stir and crystallize at 10±2°C for 6h; filter with suction, and wash the filter cake with 200mL ether; dry the filter cake at 35°C for 8h under reduced pressure, 100 g of white solid was obtained, namely amorphous atracurium cis-benzenesulfonate, with a yield of 71% and a purity of 93.3%. The XRPD pattern of amorphous cis-benzenesulfonate atracurium is shown in figure 1 shown.
[0035] (2) Preparation of new crystal form of atracurium cis-benzenesulfonate, including the following steps:
[0036] A. Dissolve 2.0 g of amorphous atracurium cis-benzene...
Embodiment 2
[0041] A new crystal form of atracurium cis-benzenesulfonate and its purification method, comprising the following steps:
[0042] (1) Preparation of amorphous atracurium cis-benzenesulfonate: Add 700mL of acetonitrile to a 2L reaction flask, stir, add 140g of crude atracurium cis-benzenesulfonate at 10±2°C, stir for 30-60min; filter , collect the filtrate; slowly add the filtrate into a 5L reaction flask containing 2800mL ether, stir and crystallize at 10±2°C for 6h; filter with suction, and wash the filter cake with 200mL ether; dry the filter cake at 35°C for 8h under reduced pressure, 100 g of white solid was obtained, namely amorphous atracurium cis-benzenesulfonate, with a yield of 71% and a purity of 93.3%. The XRPD pattern of amorphous cis-benzenesulfonate atracurium is shown in figure 1 shown.
[0043] (2) Preparation of new crystal form of atracurium cis-benzenesulfonate, including the following steps:
[0044] A. Dissolve 1.0 g of amorphous atracurium cis-benzene...
Embodiment 3
[0049] A new crystal form of atracurium cis-benzenesulfonate and its purification method, comprising the following steps:
[0050] (1) Preparation of amorphous atracurium cis-benzenesulfonate: Add 700mL of acetonitrile to a 2L reaction flask, stir, add 140g of crude atracurium cis-benzenesulfonate at 10±2°C, stir for 30-60min; filter , collect the filtrate; slowly add the filtrate into a 5L reaction flask containing 2800mL ether, stir and crystallize at 10±2°C for 6h; filter with suction, and wash the filter cake with 200mL ether; dry the filter cake at 35°C for 8h under reduced pressure, 100 g of white solid was obtained, namely amorphous atracurium cis-benzenesulfonate, with a yield of 71% and a purity of 93.3%. The XRPD pattern of amorphous cis-benzenesulfonate atracurium is shown in figure 1 shown.
[0051] (2) Preparation of new crystal form of atracurium cis-benzenesulfonate, including the following steps:
[0052] A. Dissolve 1.0 g of amorphous atracurium cis-benzene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com