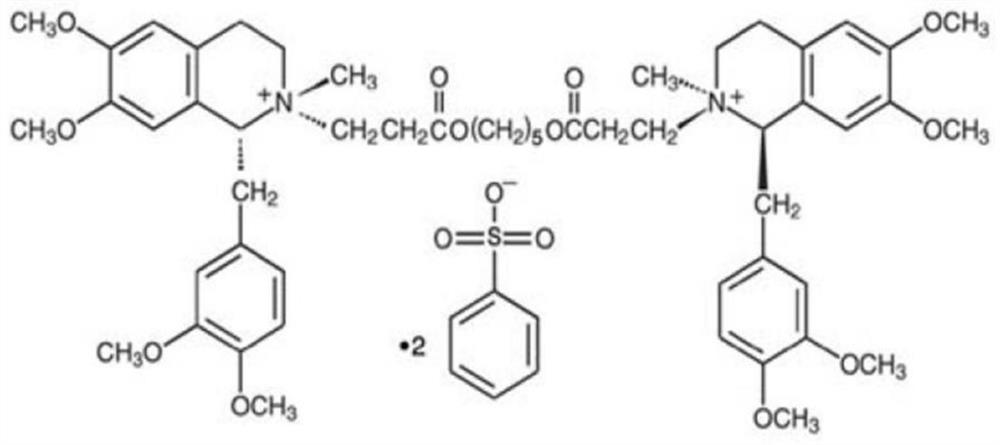
Cisatracurium besylate injection and preparation method thereof
A technology of cisatracurium besylate and injection, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve the harsh and safe conditions of production, transportation and clinical application. Sex can not be guaranteed and other problems, to achieve the effect of good compliance and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Preparation Process:
[0035] (1) Add ethanol to 800ml water for injection at 30°C, stir and mix evenly;
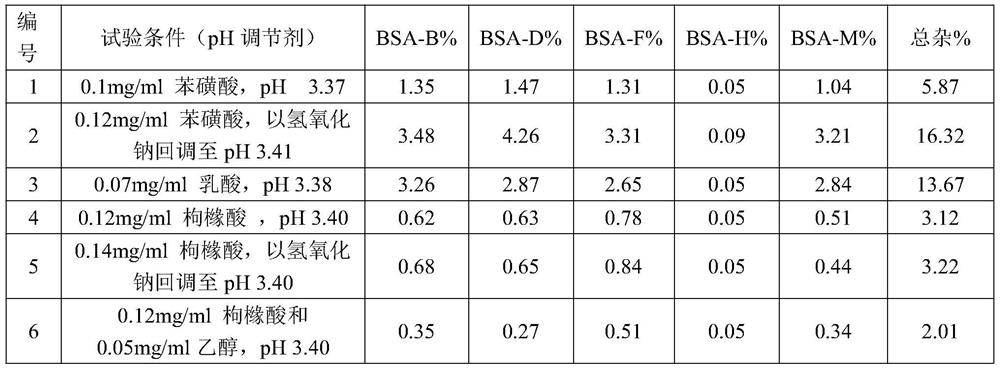
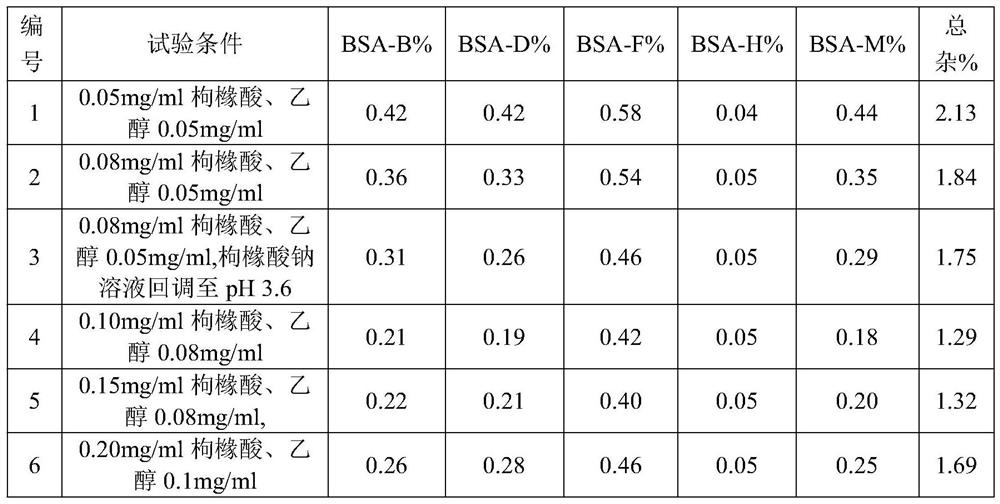
[0036] (2) Add citric acid and stir for 5 minutes to dissolve, then add cisatracurium besylate and stir for 5 minutes to dissolve; measure pH 4.51;
[0037] (3) constant volume. Dilute to 1000ml with water for injection;
[0038] (4) Sterile filtration. The liquid medicine is filtered through a 0.45 μm coarse filter and a 0.22 μm fine filter.
[0039] (5) Ampoule filling, light inspection, and packaging.
Embodiment 2
[0041]
[0042] Preparation Process:
[0043] (1) Add ethanol to 800ml water for injection at 30°C, stir and mix evenly;
[0044] (2) Add citric acid and stir for 5 minutes to dissolve, then add cisatracurium besylate and stir for 5 minutes to dissolve; measure pH3.32;
[0045] (3) constant volume. Dilute to 1000ml with water for injection;
[0046] (4) Sterile filtration. The liquid medicine is filtered through a 0.45 μm coarse filter and a 0.22 μm fine filter.
[0047] (5) Ampoule filling, light inspection, and packaging.
Embodiment 3
[0049]
[0050] Preparation Process:
[0051] (1) Add ethanol to 800ml water for injection at 30°C, stir and mix evenly;
[0052](2) Add citric acid and stir for 5 minutes to dissolve, then add cisatracurium besylate and stir for 5 minutes to dissolve; measure pH 3.01;
[0053] (3) constant volume. Dilute to 1000ml with water for injection;
[0054] (4) Sterile filtration. The liquid medicine is filtered through a 0.45 μm coarse filter and a 0.22 μm fine filter.
[0055] (5) Ampoule filling, light inspection, and packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com