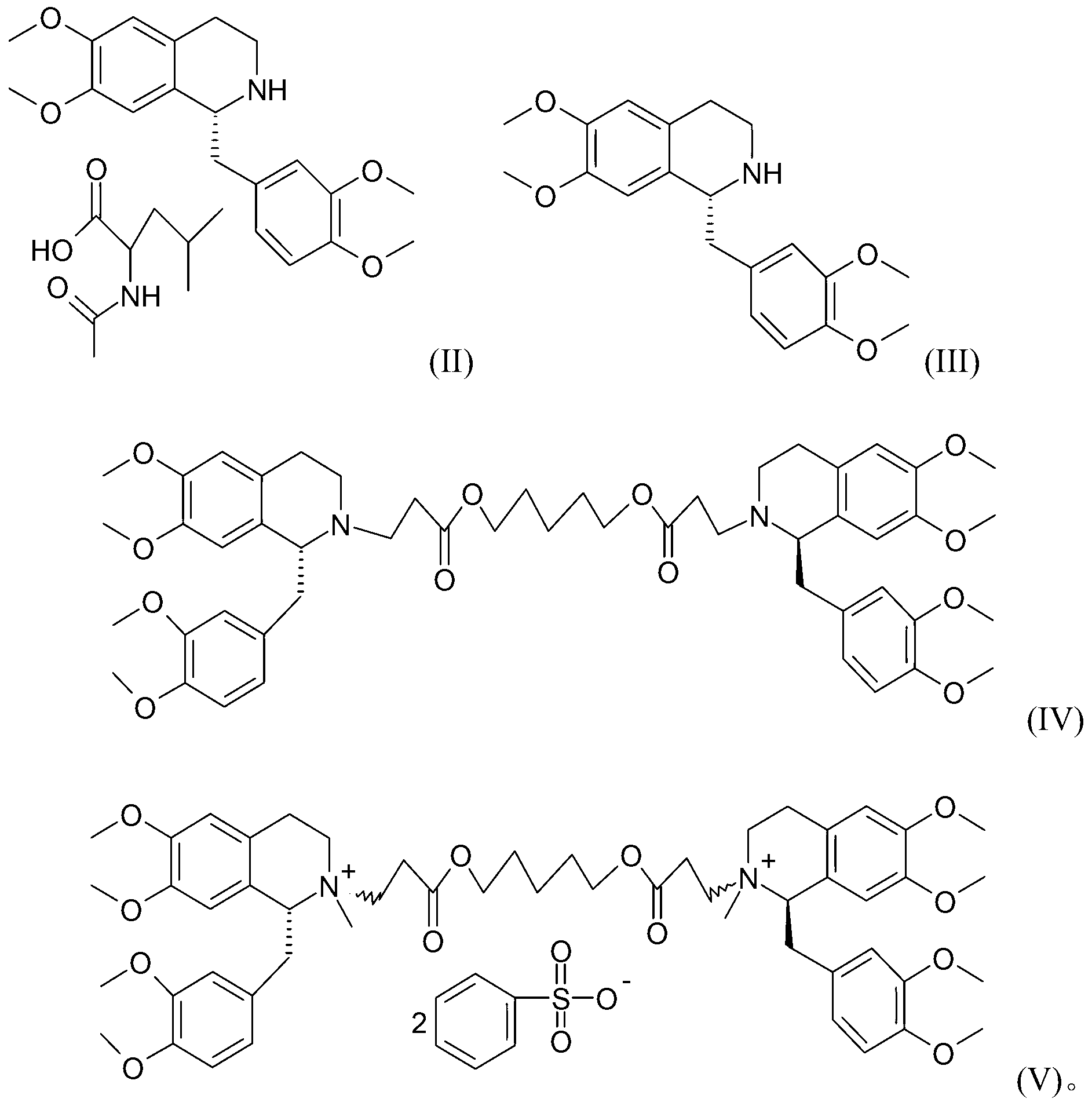
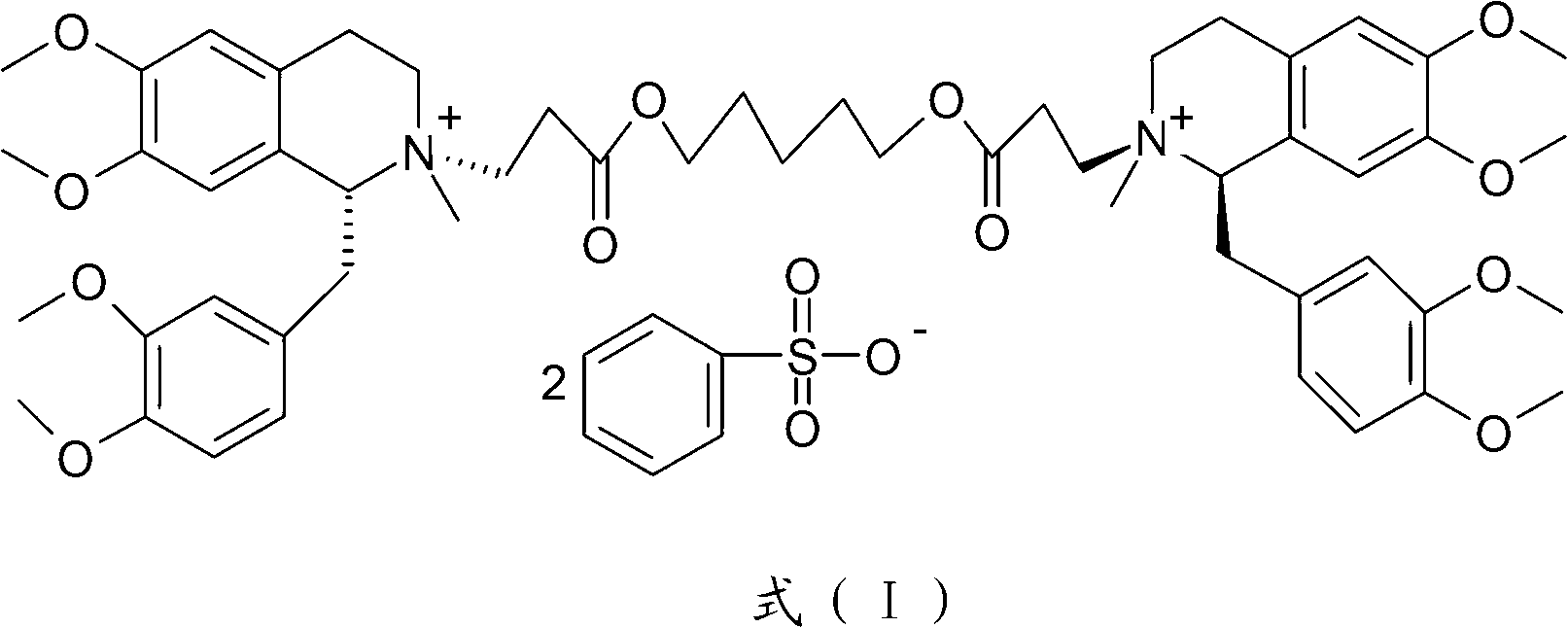
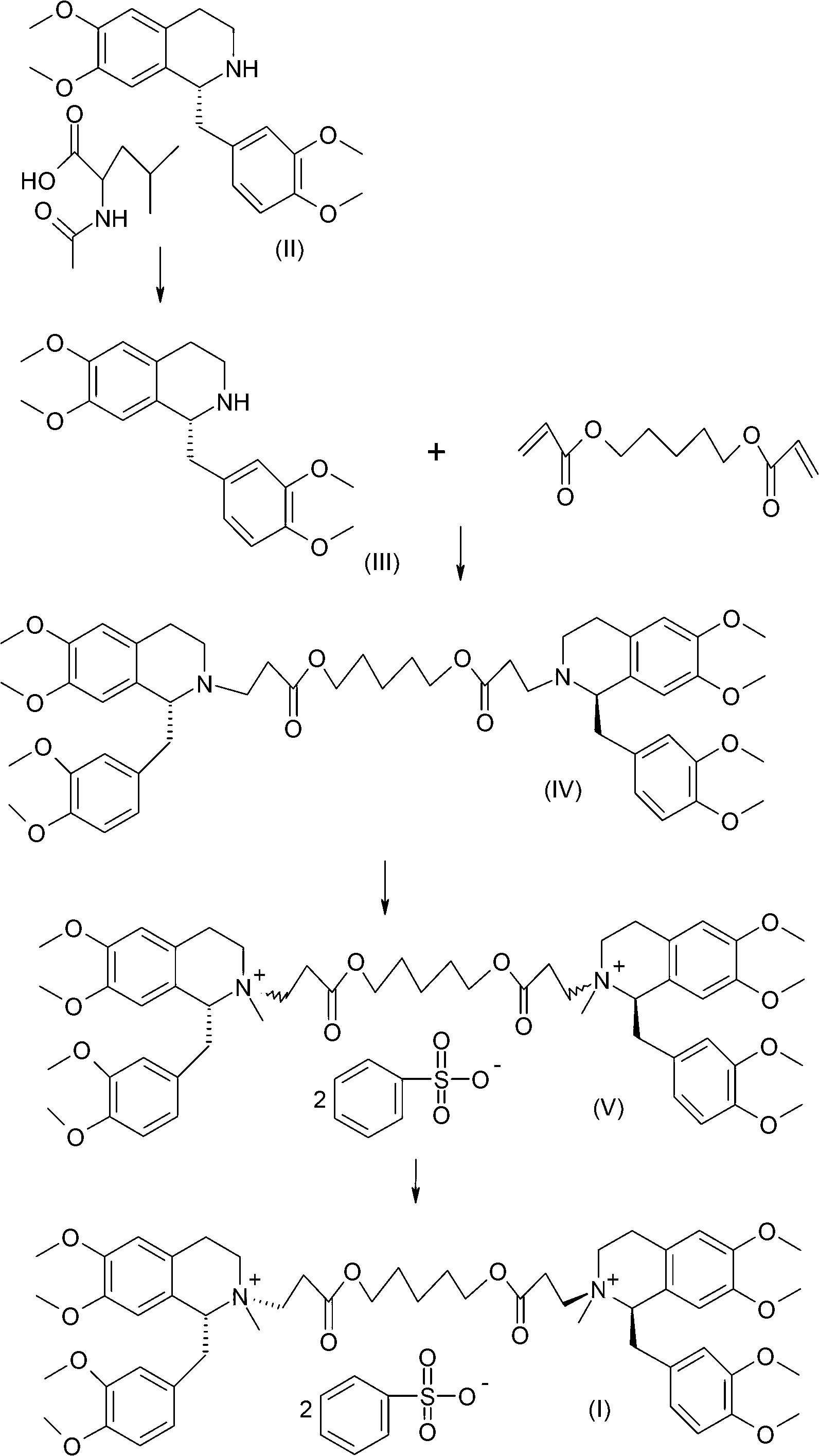
Method for preparing cisatracurium besylate
A kind of technology of atracurium cissulfonate and oxalate, applied in the direction of organic chemistry and the like, can solve the problems such as mixing of post-treatment scheme, no method for removal, and achieves reduction of product cost, improvement of quality, and improvement of yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, the preparation of compound (IV)
[0042] Put 700 g of R-tetrahydropapaverine-N-acetyl-L-leucine salt (II) into the reactor (the S type is less than 0.5%). Add 1.8L of water to dissolve. Adjust the pH to 7-8 with ammonia water. Extracted with 3.6L of dichloromethane, concentrated to dryness under high vacuum to obtain compound (III), added 143ml of compound 1,5-pentanediol diacrylate and 38ml of glacial acetic acid to react at an external temperature of 70°C for 14 hours. Reaction solution (1R,1'R)-2,2'-(3,11-dioxo-4,10-dioxa-1,13-tridecylene)-bis[1,2,3, 4-tetrahydro-6,7-dimethoxy-1-(3,4-dimethoxy)benzyl]isoquinoline (IV) HPLC purity 92.4%. Preheated in a salt-forming reactor at 45°C 6L absolute ethanol. Dilute the reaction liquid with 400ml of dichloromethane, wash it out and add it to the salt-forming reaction tank, and then add 180g of oxalic acid. Insulate and react for 2 hours, turn off the heating, and stir at room temperature for 20 hours. Fil...
Embodiment 2
[0043] Embodiment 2, the refining of compound (IV) oxalate:
[0044] Dissolve the crude oxalate wet product in 4L of water, extract with 4L of dichloromethane, concentrate to a small volume, add ethanol to continue to concentrate under reduced pressure, then add 6L of absolute ethanol, turn on cooling, stir for 20 hours, and filter to obtain (1R, 1'R)-2,2'-(3,11-dioxo-4,10-dioxa-1,13-tridecylene)-bis[1,2,3,4-tetrahydro- 6,7-Dimethoxy-1-(3,4-dimethoxy)benzyl]isoquinoline oxalate wet fine. Wet weight 1.5kg, HPLC 99.5%.
Embodiment 3
[0045] Embodiment 3, the preparation of compound (V)
[0046] Dissolve 1.5kg of wet product in 5L of water, and adjust the pH to 7-8 with ammonia water. Extract with 4 L of dichloromethane. The organic layer was dehydrated and concentrated below 60°C to an oil. Cool down to below 30°C, add 450ml of methyl benzenesulfonate, 700ml of acetonitrile, and 1.55g of anhydrous sodium carbonate. Reaction at an external temperature of 28-30°C for 20 hours. Diluted with 2L of dichloromethane and added dropwise to 20L of anhydrous ether. Filter and vacuum dry for 24 hours to obtain 632 g of 1R, 1'R-atracurium benzenesulfonate. The HPLC purity of 3 chiral isomers is 97.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com