Cisatracurium besylate lyophilized preparation composition stable at room temperature and preparation method thereof
A technology for cisatracurium besylate and freeze-dried preparations, which is applied in the field of cisatracurium besylate freeze-dried preparations and its preparation, and can solve the problem of increased production costs, unfavorable storage for hospitals and patients, etc. problems, to achieve the effect of facilitating production, storage and carrying, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
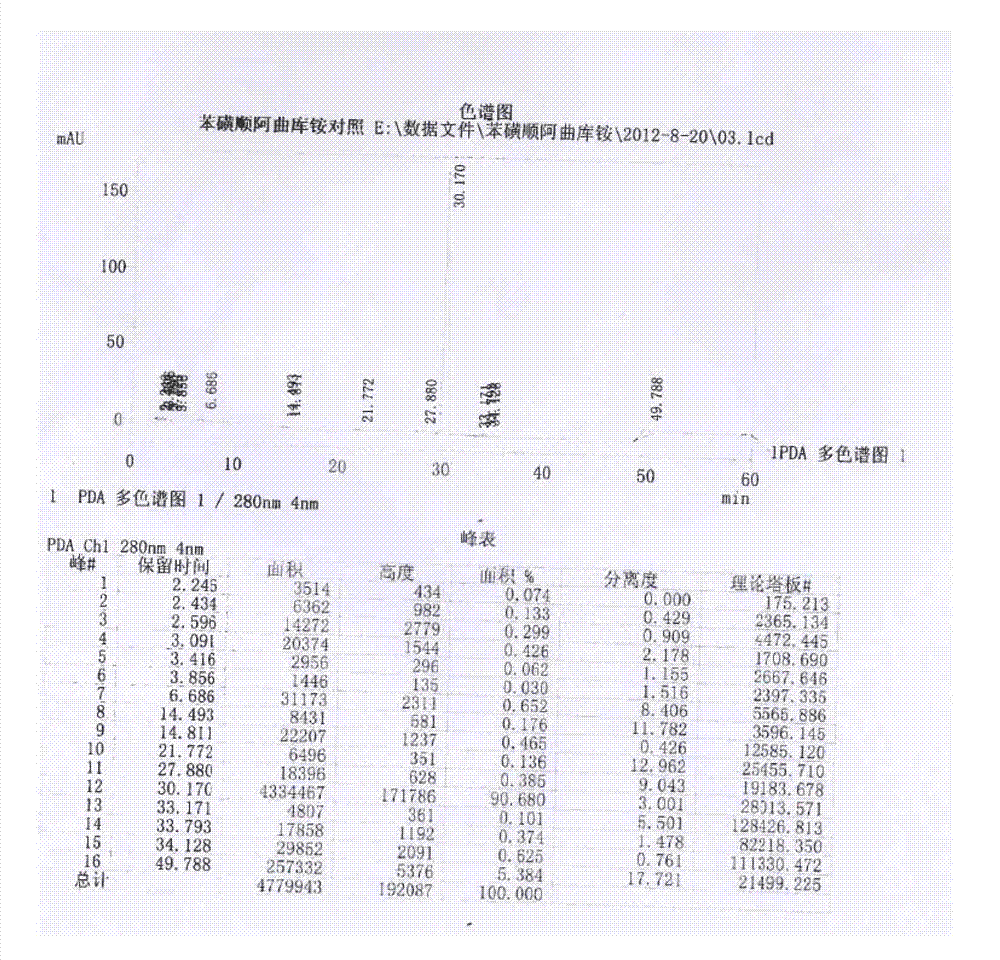
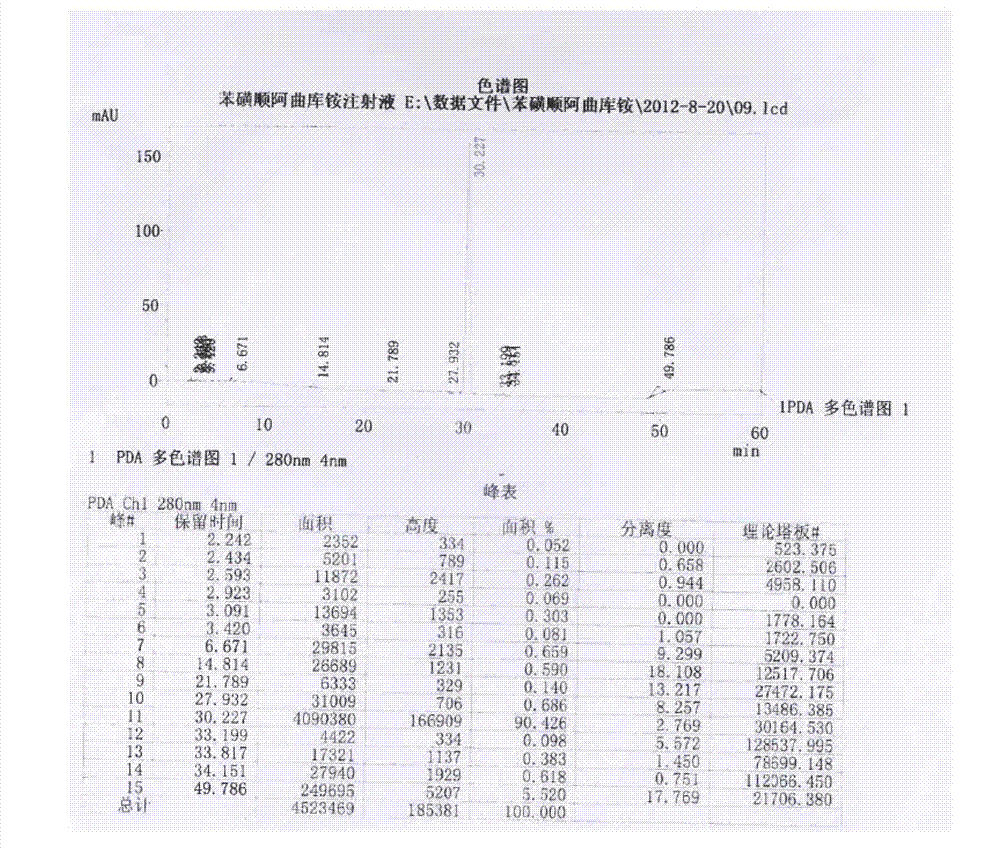
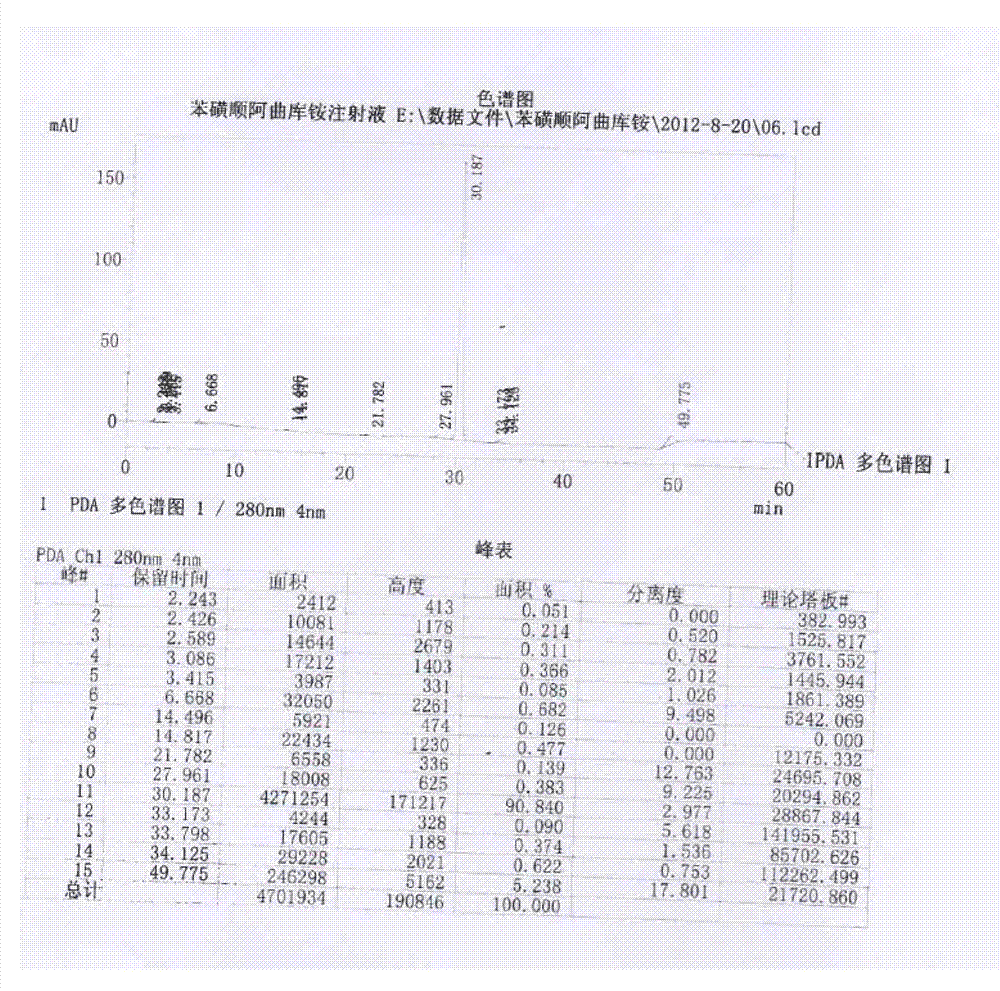
Image
Examples
Embodiment 1
[0026] Prescription:
[0027]
[0028] Preparation Process:
[0029] Take about 80% of the total amount of water for injection, control the water temperature at 25°C±5°C, add 30g of precisely weighed injection-grade lactose, and 10g of cisatracurium besylate, fully stir until completely dissolved, and measure the pH value. Add benzenesulfonic acid aqueous solution dropwise, control the pH value to about 3.5, add water for injection to the full amount, add 0.1% activated carbon for needles, stir for 30 minutes at 25°C±5°C, sterilize and filter the solution with a 0.22μm microporous filter membrane, and distribute the solution in 7m1 In the standard vials, the filling capacity of each bottle is 5ml.
[0030] Put the prepared vials filled with cisatracurium besylate into a freeze-drying box, and then reduce the temperature in the freeze-drying machine to below -45°C within 2 hours to make it freeze quickly. Vacuumize and make the atmospheric pressure in the box reach 2.66pa...
Embodiment 2
[0032] Prescription:
[0033]
[0034] Preparation Process:
[0035] Take about 80% of the total amount of water for injection, control the water temperature at 25°C ± 5°C, add 40g of precisely weighed mannitol, and 10g of cisatracurium besylate, fully stir until completely dissolved, measure the pH value, drop Add benzenesulfonic acid aqueous solution, control the pH value to be about 3.5, add water for injection to the full amount, add 0.1% activated carbon for needles, stir for 30 minutes at 25°C±5°C, sterilize and filter the solution with a 0.22μm microporous membrane, and pack the solution in 7m1 size In the vials, the volume of each bottle is 5ml.
[0036] Put the prepared vials filled with cisatracurium besylate into a freeze-drying box, and then reduce the temperature in the freeze-drying machine to below -45°C within 2 hours to make it freeze quickly. Vacuumize and make the atmospheric pressure in the box reach 2.66pa within 30 minutes. Dry according to the tem...
Embodiment 3
[0038] Prescription:
[0039]
[0040] Preparation Process:
[0041]Take about 80% of the total amount of water for injection, control the water temperature at 25°C ± 5°C, add 30g of precisely weighed glycine, and 10g of cisatracurium besylate, fully stir until completely dissolved, measure the pH value, add dropwise Aqueous solution of benzenesulfonic acid, control the pH value to about 3.5, add water for injection to the full amount, add 0.1% activated carbon for needles, stir for 30 minutes at 25°C±5°C, sterilize and filter the solution with a 0.22μm microporous filter membrane, and pack the solution in 7m1 size containers In vials, the volume of each bottle is 5ml.
[0042] Put the prepared vials filled with cisatracurium besylate into a freeze-drying box, and then reduce the temperature in the freeze-drying machine to below -45°C within 2 hours to make it freeze quickly. Vacuumize and make the atmospheric pressure in the box reach 2.66pa within 30 minutes. Dry acco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com