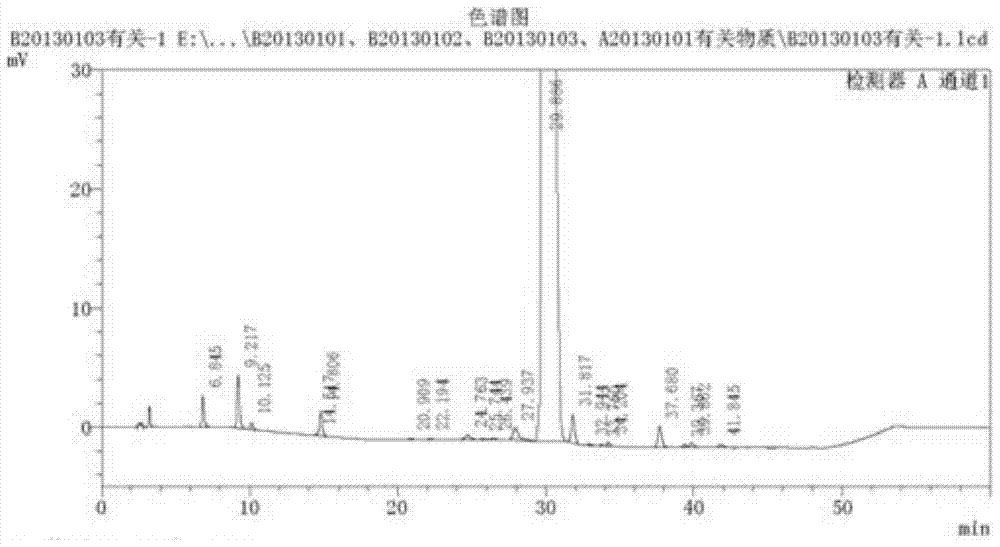
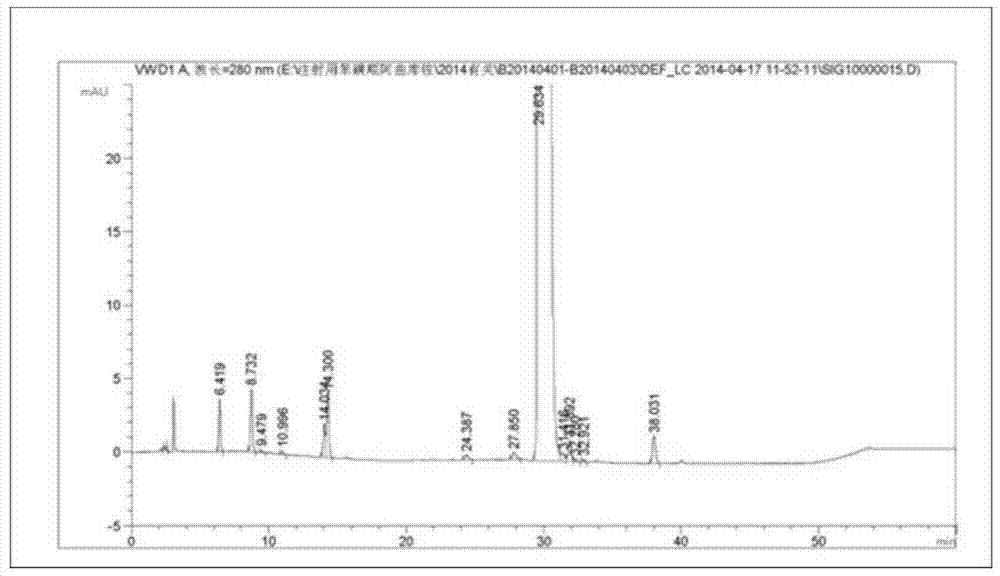
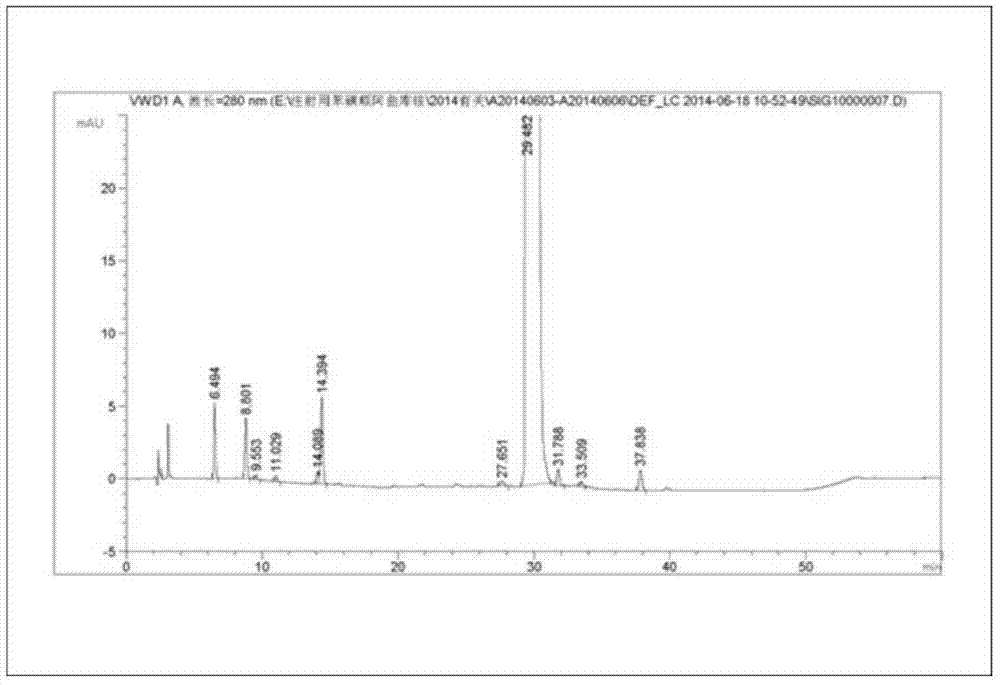
Production process of cisatracurium besylate for injection for storage and transportation at 25°C
A technology for cisatracurium besylate and injection is applied in the field of cisatracurium besylate for injection and its preparation and freeze-drying production process, and can solve the problems of difficult industrialization, high price, poor stability and the like , to achieve the effect of more industrialized production, stable product quality and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0036]
[0037]
[0038] Preparation process: Put 70% of the prescribed amount of water for injection, control the water temperature at 25°C, add 10 mg of cisatracurium besylate, 50 mg of mannitol, and 50 mg of dextran-20, which are precisely weighed, stir well to dissolve them, and measure pH value, add 20% benzenesulfonic acid solution to adjust the pH value to 3.0, add water for injection to the full amount, add 0.05% activated carbon, stir well to make it uniform, sterilize and filter through 0.45μm and 0.22μm microporous membranes, and divide the solution In a controlled vial of 7m1 size, the filling volume is 3ml / bottle.
[0039] Freeze drying process:
[0040] (1) Pre-freezing: Put the filled medicine on the partition of the freeze-drying box, and the temperature of the partition is rapidly lowered to -45°C, and kept for 3 hours to completely freeze;
[0041] (2) Sublimation drying: The temperature of the partition is -45°C, the vacuum control is less than 30Pa, ...
Embodiment B
[0045]
[0046]
[0047] Preparation process: Put 75% of the prescribed amount of water for injection, control the water temperature at 35°C, add 20 mg of cisatracurium besylate, 50 mg of mannitol, and 50 mg of dextran-20, which are precisely weighed, stir well to dissolve them, and measure For the pH value, add 20% benzenesulfonic acid solution to adjust the pH value to 4.0. Add water for injection to the full amount, add 0.1% activated carbon, stir well to make it uniform, sterilize and filter through 0.45μm and 0.22μm microporous membranes, and divide the solution In a controlled vial of 7m1 size, the filling volume is 2ml / bottle.
[0048]Freeze drying process:
[0049] (1) Pre-freezing: Put the filled medicine on the partition of the freeze-drying box, and the temperature of the partition is rapidly lowered to -50°C, and kept for 4 hours to completely freeze;
[0050] (2) Sublimation drying: The temperature of the partition is -50°C, the vacuum control is less than ...
Embodiment C
[0054]
[0055]
[0056] Preparation process:
[0057] Add 73% of the prescribed amount of water for injection, control the water temperature at 32°C, add 16 mg of cisatracurium besylate, 50 mg of mannitol, and 50 mg of dextran-20 that are precisely weighed, fully stir to dissolve it, and measure the pH value. Add a solution containing 20% benzenesulfonic acid, adjust the pH value to 3.0, add water for injection to the full amount, add 0.56% activated carbon, stir well to make it uniform, sterilize and filter through 0.45 μm and 0.22 μm microporous membranes, and distribute the solution In a controlled vial of 7m1 size, the filling volume is 2.4ml / bottle.
[0058] Freeze drying process:
[0059] (1) Pre-freezing: Put the filled medicine on the partition of the freeze-drying box, and the temperature of the partition is rapidly cooled to -48°C, and kept for 3.5 hours to completely freeze;
[0060] (2) Sublimation drying: The temperature of the partition is -48°C, the v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com