Preparation method of high-purity cisatracurium besylate
A high-purity technology of atracurium cis-benzenesulfonate, which is applied in the field of preparation of high-purity atracurium cis-benzenesulfonate, can solve problems such as excitatory effects, and achieve high product purity, small side reactions, and low price Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
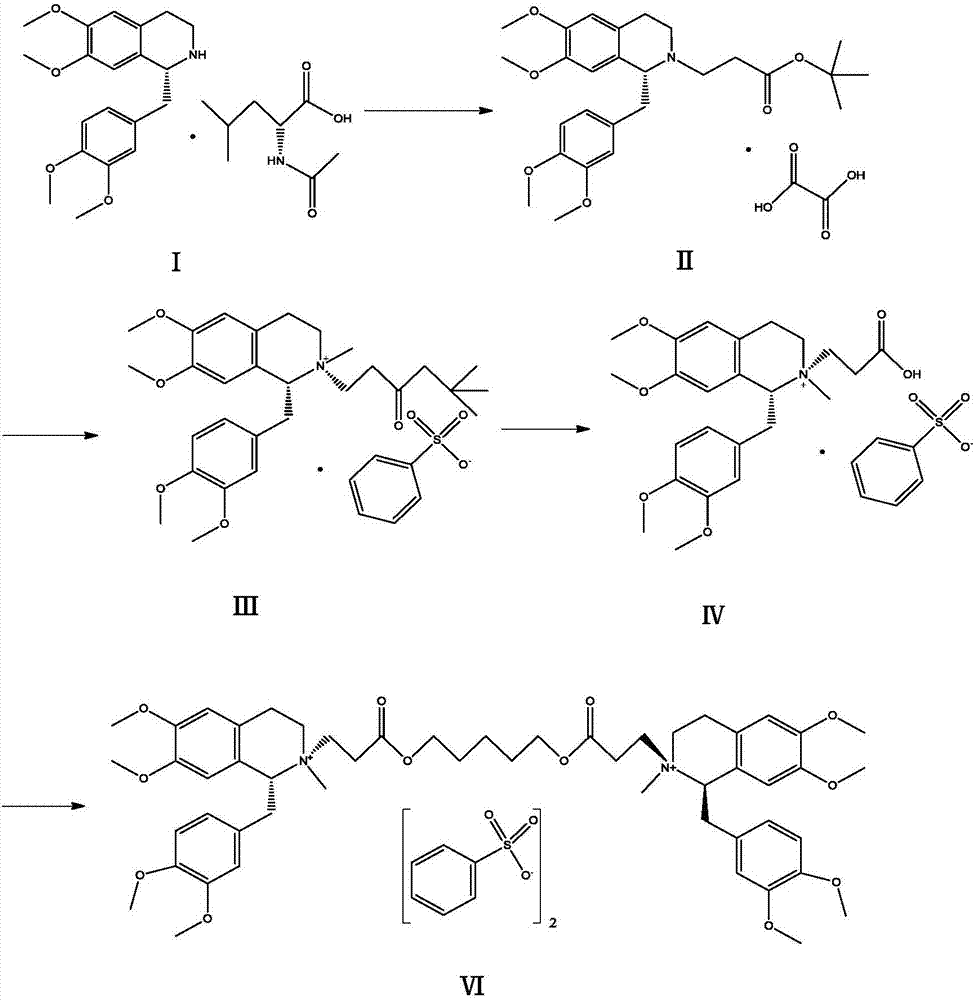
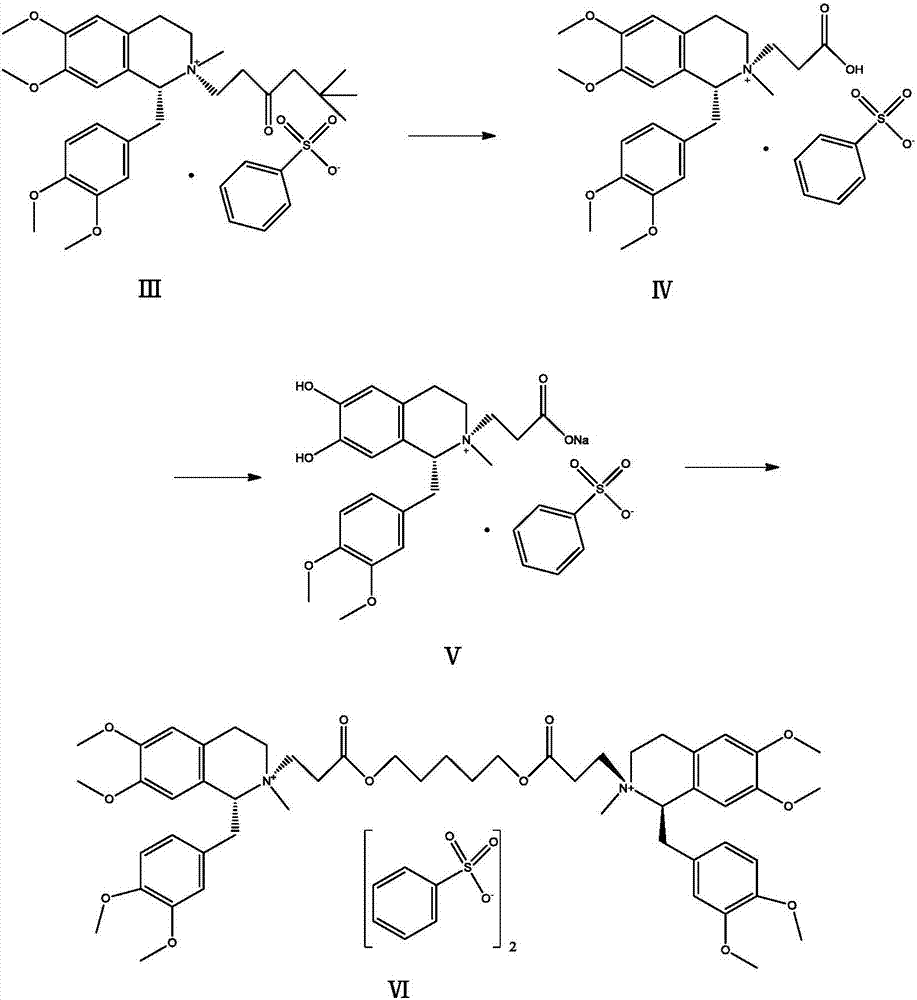
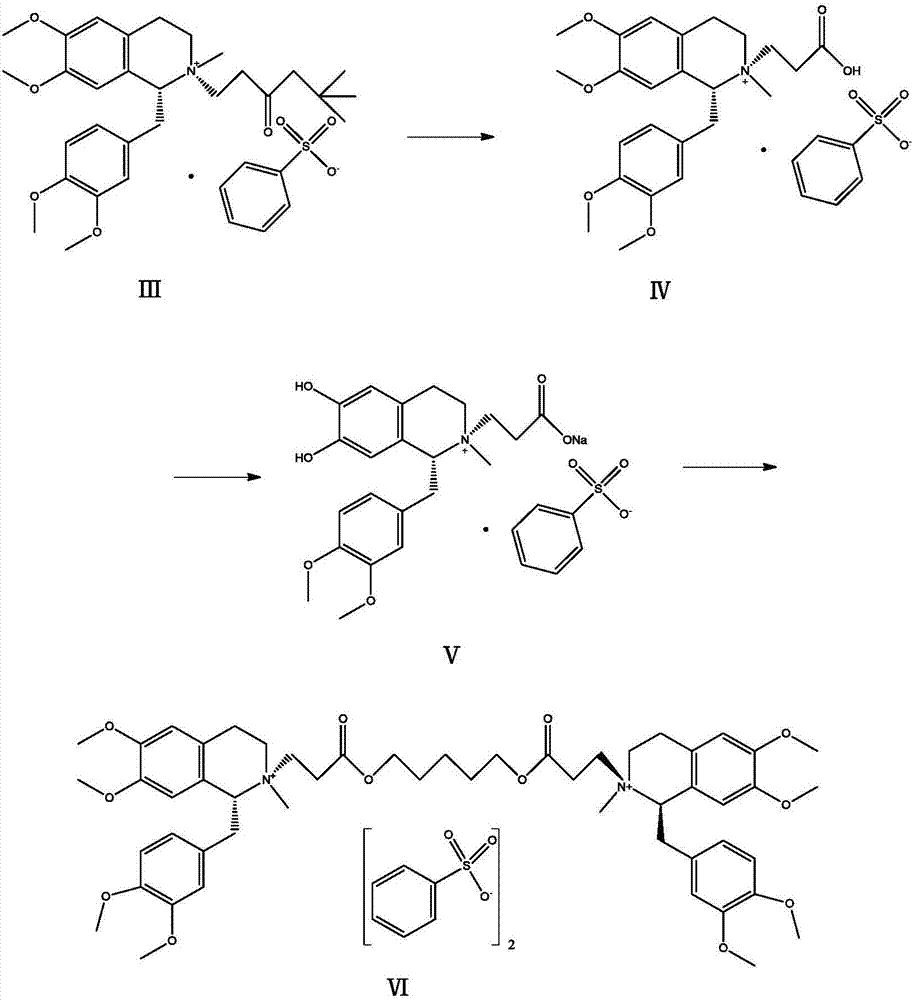
[0020] Add 6g of the compound of formula (Ⅲ) and 6ml of 70% benzenesulfonic acid into a 50ml reaction bottle, heat in a water bath, keep the reaction at 50°C for 2h, and cool to room temperature. Add 20ml of acetonitrile and 0.8g of sodium bicarbonate into the reaction flask, react at a temperature below 50°C for 30min, cool and crystallize to obtain the compound of formula (V) as a solid. Add the compound of formula (Ⅴ) into a 100ml reaction flask, add 60ml of dichloromethane, then weigh 1.3g of 1,5-dichloropentane into the reaction flask, start stirring, and heat to reflux for 5h. The feed solution was dropped into 240ml of anhydrous ether with stirring, and the solid was precipitated, filtered, washed with anhydrous ether, and dried in vacuo to obtain 3.96 g of cisatracurium besylate, with a yield of 68.7% and a purity of 99.2%.
Embodiment 2
[0022] Add 6g of the compound of formula (Ⅲ) and 6ml of 70% benzenesulfonic acid into a 50ml reaction bottle, heat in a water bath, keep the reaction at 50°C for 2h, and cool to room temperature. Add 20ml of acetonitrile and 0.4g of sodium hydroxide into the reaction flask, react at a temperature below 50°C for 30min, cool and crystallize to obtain the compound of formula (V) as a solid. Add the compound of formula (Ⅴ) into a 100ml reaction flask, add 60ml of dichloromethane, weigh 1.3g of 1,5-dichloropentane into the reaction flask, start stirring, and heat to reflux for 5h. The feed solution was dripped into 240ml of anhydrous diethyl ether with stirring, and the solid was precipitated, filtered, washed with anhydrous diethyl ether, and dried in vacuo to obtain 3.85 g of cisatracurium besylate with a yield of 66.8% and a purity of 99.0%.
Embodiment 3
[0024] Add 6g of the compound of formula (III) and 6ml of 70% benzenesulfonic acid into a 50ml reaction bottle, heat in a water bath, keep the reaction at 50°C for 2h, and cool to room temperature. Add 20ml of acetonitrile and 0.8g of sodium bicarbonate into the reaction flask, react at a temperature below 30°C for 30min, cool and crystallize to obtain the compound of formula (V) as a solid. Add the compound of formula (Ⅴ) into a 100ml reaction flask, then add 60ml of dichloromethane, weigh 1.3g of 1,5-dichloropentane into the reaction flask, start stirring, and heat to reflux for 5h. The feed solution was dripped into 240ml of anhydrous diethyl ether with stirring, and the solid was precipitated, filtered, washed with anhydrous diethyl ether, and dried in vacuo to obtain 3.79 g of cisatracurium besylate, with a yield of 65.7% and a purity of 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com