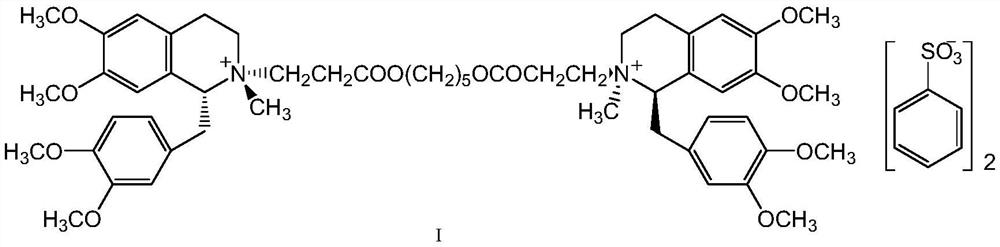
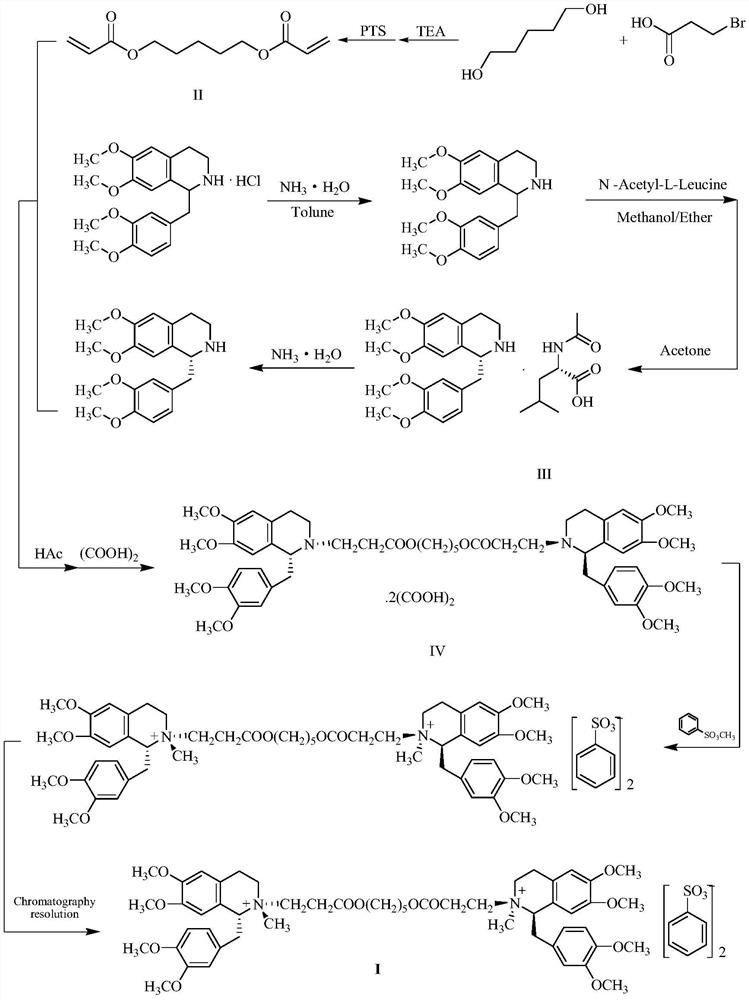
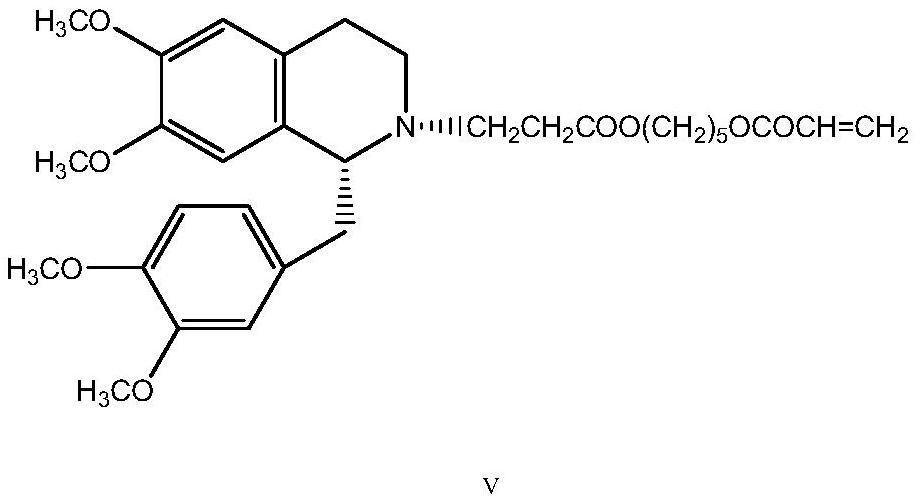
A kind of preparation method of besylate cisatracurium intermediate
A technology of cisatracurium besylate and an intermediate, which is applied in the field of pharmaceutical synthesis, can solve the problems of complicated post-processing, doping chloride ions, affecting the yield of formula II, etc., and achieves easy industrial production, simple operation steps, and low post-processing Handling simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of formula VI compound
[0028] R-tetrahydropapaverine-N-acetyl-L-leucine salt (III) 103.3g (0.2mol) was dissolved in 1000ml of water, then 18.6ml of concentrated ammonia water (molar concentration 13mol / L) was added and stirred for 30min. Extract twice with toluene, 500ml each time. The toluene extracts were combined, washed with 600ml of water, the organic phase was dried with anhydrous sodium sulfate, filtered, washed with a small amount of toluene, and the filtrate was concentrated to dryness under reduced pressure in a 65°C water bath.
[0029] Add 30.8 g (0.24 mol) of tert-butyl acrylate and 6.9 ml (0.12 mol) of acetic acid to the product, and react at 75° C. for 6 hours. After the reaction was completed, 100 ml of acetone was added to dissolve, and then a mixture obtained by dissolving 26.5 g (0.21 mol) of oxalic acid dihydrate in 850 ml of acetone was added and stirred for 30 min. After crystallization at room temperature overnigh...
Embodiment 2
[0030] Embodiment 2: the preparation of formula IV compound
[0031] 56.2 g (0.1 mol) of the compound of formula VI was dissolved by adding 550 ml of water, 17.2 ml of concentrated ammonia water was added, and the reaction was stirred for 30 min. Extract twice with toluene, 200ml each time. The toluene extracts were combined, washed with 400ml of water, the organic phase was dried with anhydrous sodium sulfate, filtered, washed with a small amount of toluene, and the filtrate was concentrated to dryness under reduced pressure in a 65°C water bath.
[0032] Add 45.2 g (0.2 mol) of 70% benzenesulfonic acid to the product, and react at 50-55° C. for 4 hours. After the reaction was completed, concentrate under reduced pressure, add 400ml of dichloromethane to dissolve, then add 4.73g (0.045mol) of 1,5-pentanediol, and reflux for 6 hours of water separation. After the reaction is finished, wash with water 3 times, each 400ml. Add 200ml of water to the organic phase, add 7.6ml of...
Embodiment 3
[0033] Embodiment 3: the preparation of formula IV compound
[0034] 56.2 g (0.1 mol) of the compound of formula VI was dissolved by adding 550 ml of water, 17.5 ml of concentrated ammonia water was added, and the reaction was stirred for 30 min. Extract twice with toluene, 200ml each time. The toluene extracts were combined, washed with 400ml of water, the organic phase was dried with anhydrous sodium sulfate, filtered, washed with a small amount of toluene, and the filtrate was concentrated to dryness under reduced pressure in a 65°C water bath.
[0035]The product was added to a mixed solution consisting of 38g (0.2mol) of p-toluenesulfonic acid monohydrate dissolved in 50ml of water, and reacted at 50-55°C for 4.5 hours. After the reaction is completed, concentrate under reduced pressure, add 400ml of dichloromethane to dissolve, then add 4.73g (0.045mol) of 1,5-pentanediol, and reflux for 5.5 hours to separate water. After the reaction is finished, wash with water 3 tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com