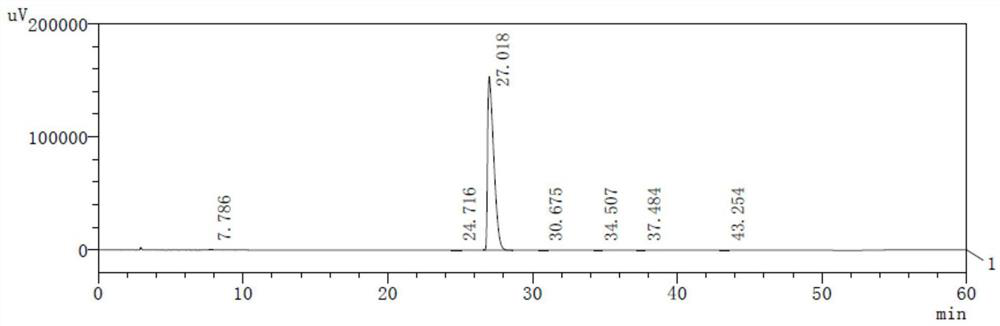
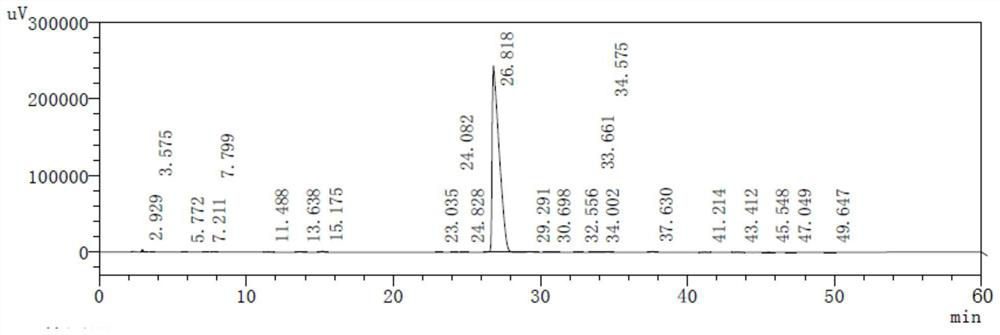
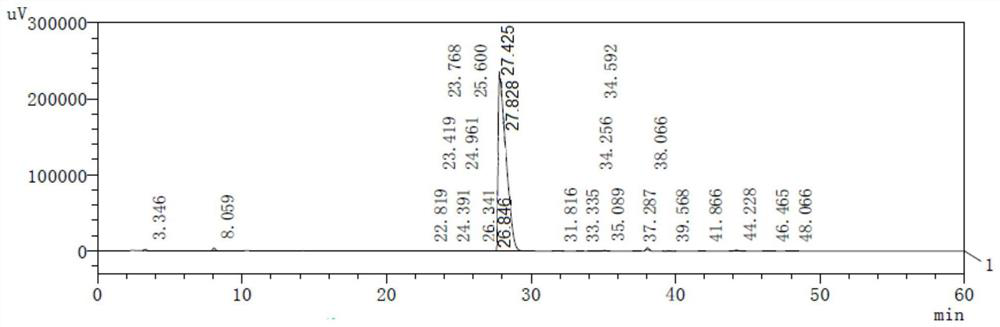
Preparation method and application of cisatracurium besilate
A technology of cisatracurium besylate and reaction technology, which is applied in the field of preparation of cisatracurium besylate, can solve the problem of complex preparation of pentanediol diacrylate, reaction yield of less than 60%, large solvent consumption, etc. problems, to achieve the effect of easier control of residues, fewer types of impurities and lower costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The present embodiment provides a kind of preparation method of cisatracurium besylate, and the reaction formula is as follows:
[0061]
[0062] Specific steps are as follows:
[0063] (1) Take R-tetrahydropapaverine-N-acetyl-L-leucine salt (50g, 0.0967mol) in toluene (250mL) and water (250mL) and use 20% sodium carbonate aqueous solution to adjust the pH to 9, divide liquid, the organic layer was concentrated under reduced pressure at 55°C until no dripping; then added acetonitrile (250mL) to dissolve, then added methyl 3-bromopropionate (19.4g, 0.116mol) and anhydrous sodium carbonate (12.3g, 0.116mol ), reacted at 80°C for 24h; after the reaction was completed, cooled to 20°C and filtered; the filtrate was concentrated under reduced pressure at 40°C until no dripping, added acetone (500mL) to dissolve, added oxalic acid (10.4g, 0.116mol) to form salt and crystallize , the wet product was dried to obtain Intermediate A (47.7g), yield: 95.0%;
[0064] (2) Take th...
Embodiment 2
[0076] The present embodiment provides a kind of preparation method of cisatracurium besylate, and the reaction formula is as follows:
[0077]
[0078] Specific steps are as follows:
[0079](1) Take R-tetrahydropapaverine-N-acetyl-L-leucine salt (50g, 0.0967mol) in toluene (250mL) and water (250mL) and use triethylamine to adjust the pH to 8, separate the liquids, The organic layer was concentrated under reduced pressure at 55°C until it did not drop; then acetonitrile (250mL) was added to dissolve, and then methyl 3-bromopropionate (16.2g, 0.0967mol) and triethylamine (9.8g, 0.0967mol) were added, and in React at 40°C for 28 hours; after the reaction is completed, cool to 20°C and filter; the filtrate is concentrated under reduced pressure at 40°C until it does not drop, add acetone (500mL) to dissolve, add oxalic acid (10.4g, 0.116mol) to form a salt and crystallize, wet product Intermediate A (47.1g) was obtained after drying, yield: 93.86%;
[0080] (2) Take the int...
Embodiment 3
[0084] The present embodiment provides a kind of preparation method of cisatracurium besylate, and the reaction formula is as follows:
[0085]
[0086] Specific steps are as follows:
[0087] (1) Take R-tetrahydropapaverine-N-acetyl-L-leucine salt (50g, 0.0967mol) in toluene (250mL) and water (250mL) to adjust the pH to 10 with aqueous sodium hydroxide solution, and separate the liquid , the organic layer was concentrated under reduced pressure at 55°C until it did not drop; then acetonitrile (250 mL) was added for dissolution, and then methyl 3-bromopropionate (22.6 g, 0.1354 mol) and sodium hydroxide (5.4 g, 0.1354 mol) were added, React at 90°C for 20h; after the reaction is complete, cool to 20°C and filter; the filtrate is concentrated under reduced pressure at 40°C until it does not drop, add acetone (500mL) to dissolve, add oxalic acid (10.4g, 0.116mol) to form a salt and crystallize, wet After drying the product, intermediate A (46.3g) was obtained, yield: 92.0%; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com