3-hydroxy olefine acid derivative and its preparation and application
A technology for hydroxyalkenoic acid and derivatives, which is applied in the field of 3-hydroxyalkenic acid derivatives and its preparation and application, which can solve the problems of low efficiency, unfavorable preparation, inconvenient preparation of intermediates, etc., to ensure optical purity and enrich compounds Kind of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
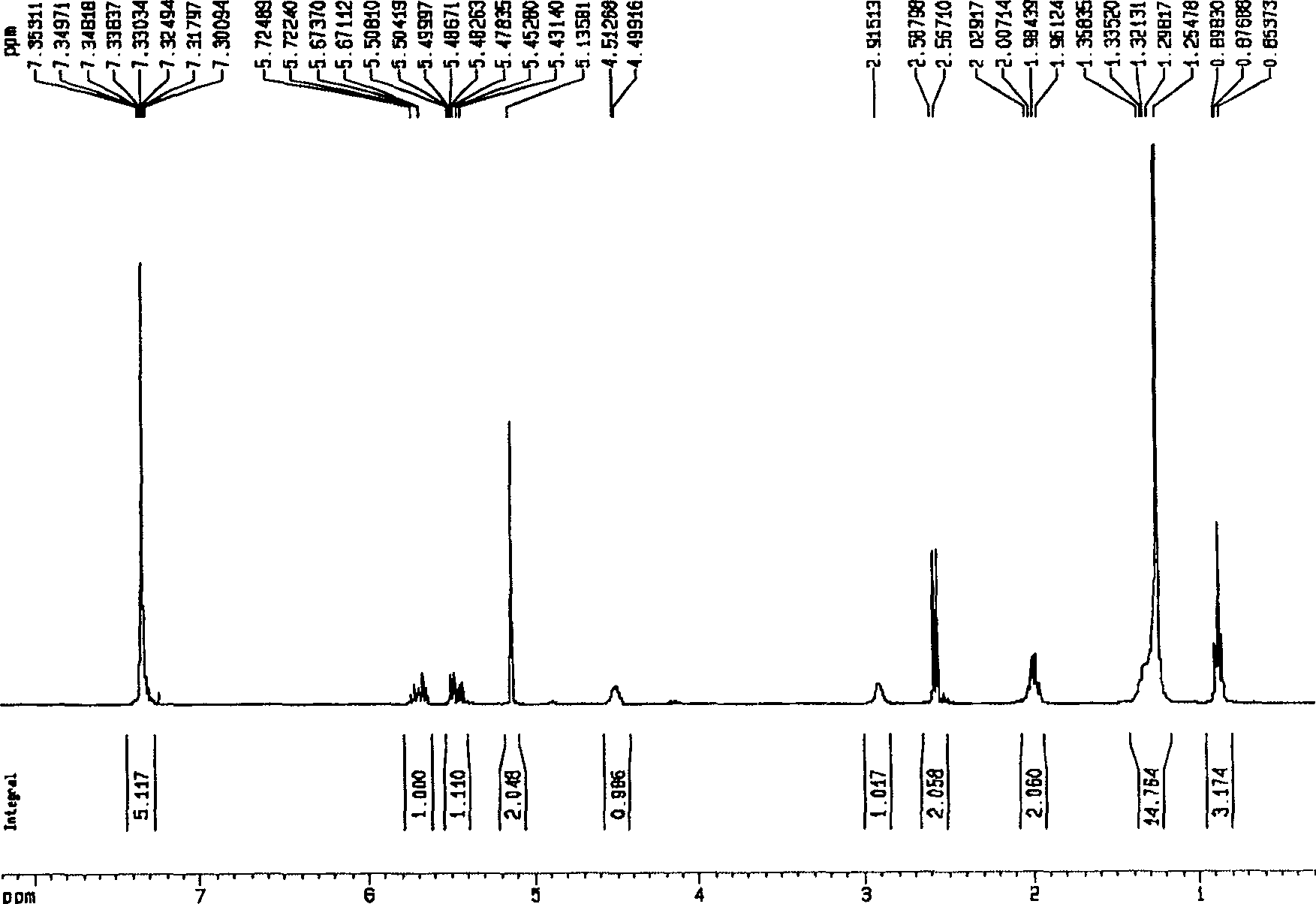
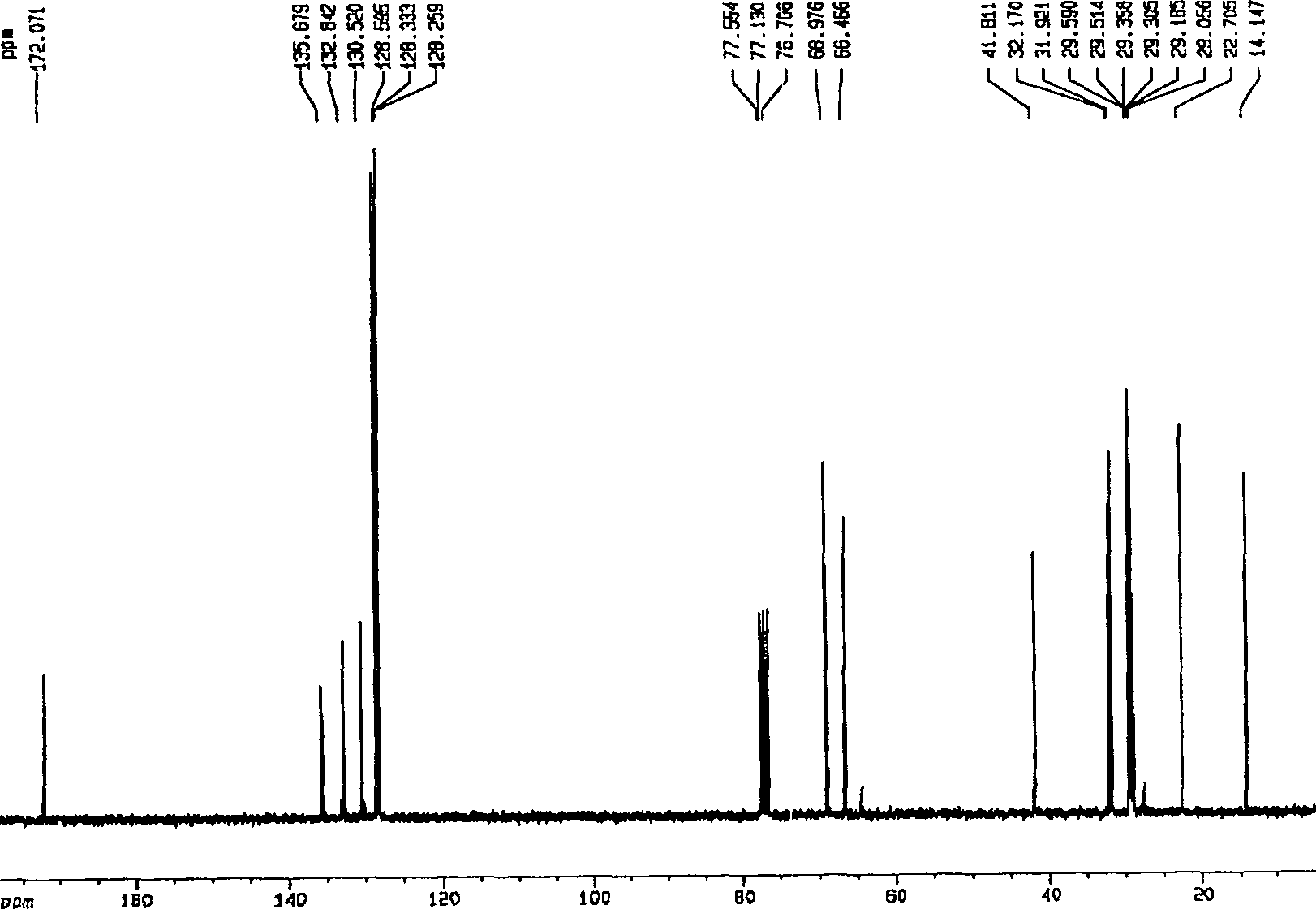
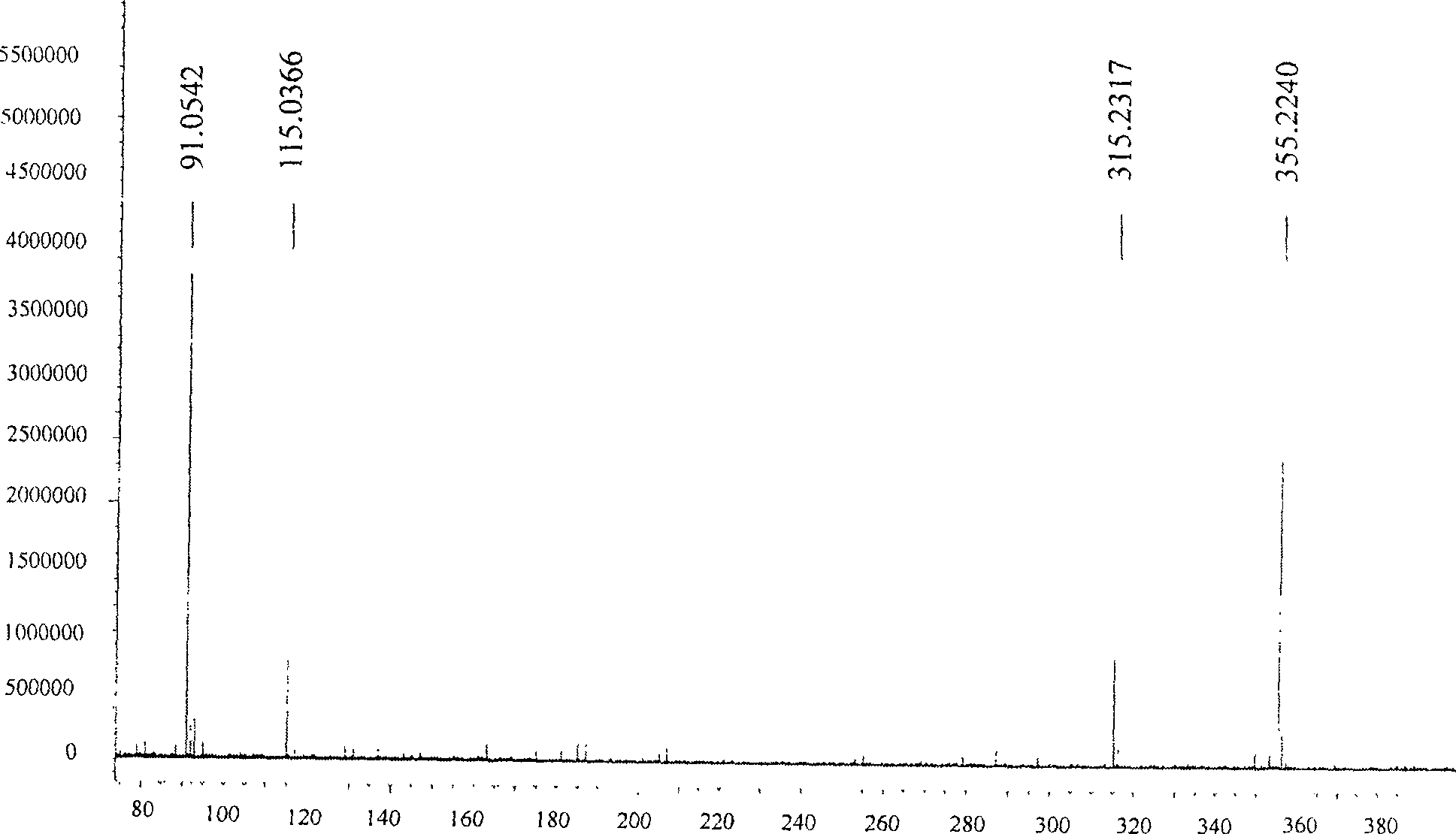
[0052] Embodiment 1, the synthesis of (S)-3-hydroxyl-4-pentenoic acid benzyl ester
[0053]
[0054] Add triethylamine (2.11 mL, 1.5 equiv.) to a solution of crude (S)-3-hydroxy-4-pentenoic acid (1.16 g, 10 mmol) in acetone (15 mL), cool to 0°C, and slowly drop Add benzyl bromide (1.45 mL, 1.2 equiv.), react at room temperature for 12 hours, concentrate and purify by column chromatography (silica gel column, petroleum ether: ethyl acetate volume ratio is 7:1-5:1 as mobile phase) (purchased from silica gel From Qingdao Ocean Chemical Co., Ltd., petroleum ether (60-90° C.) and ethyl acetate were purchased from Beijing Chemical Reagent Company) to obtain 1.70 g of oil, with a yield of 82.4%.
[0055] [ α ] D 15 = - 2.06 ( c 2.92 , CH 2 Cl...
Embodiment 2
[0058] Embodiment 2, the synthesis of (S)-3-O-acetyl-4-pentenoic acid benzyl ester
[0059]
[0060] A mixture of (S)-benzyl 3-hydroxy-4-pentenoate (206 mg, 1.0 mmol), pyridine (0.242 ml, 3 equiv.) and acetic anhydride (0.142 ml, 1.5 equiv) was stirred at room temperature Overnight, diluted with ethyl acetate, washed with water three times, dried over anhydrous sodium sulfate, concentrated, and purified by column chromatography (silica gel column, petroleum ether: ethyl acetate volume ratio of 50:1 as mobile phase) (silica gel was purchased from Qingdao Haiyang Chemical Co., Ltd., petroleum ether (60-90° C.) and ethyl acetate were purchased from Beijing Chemical Reagent Company), and 240.2 mg of the product were obtained with a yield of 96%.
[0061] [ α ] D 15 = - 1.04 ( c = 1.43 , ...
Embodiment 3
[0064] Embodiment 3, the synthesis of (S)-3-O-tert-butyldimethylsilyl-4-pentenoic acid benzyl ester
[0065]
[0066] Dissolve (S)-3-hydroxy-4-pentenoic acid benzyl ester (154.5 mg, 0.75 mmol) and imidazole (128 mg, 1.88 mmol) in 2.5 ml of N, N-dimethylformamide, and add Add tert-butyldimethylsilyl chloride (147 mg, 0.97 mmol) in batches, add 20 ml of water after 6 hours, extract with ethyl acetate, concentrate the extract after drying over anhydrous sodium sulfate, and purify by column chromatography (silica gel column, The volume ratio of petroleum ether: ethyl acetate is 50:1 as the mobile phase) (silica gel was purchased from Qingdao Ocean Chemical Co., Ltd., petroleum ether (60-90 ° C) and ethyl acetate were purchased from Beijing Chemical Reagent Company), and 155 mg of the product was obtained. Yield 87.5%.
[0067] [ α ] D 27 = - 1.70 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com