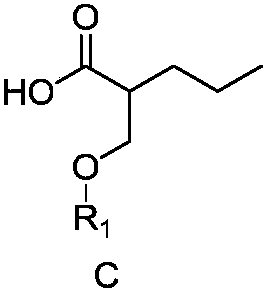
Preparation method for brivaracetam intermediate
A technology of intermediates and carboxylic acid compounds, applied in the field of preparation of brivaracetam intermediates, can solve the problem of low utilization rate of isomeric intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Potassium tert-butoxide (5.6 g, 0.05 mol) was dissolved in 120 mL THF. The reaction solution was cooled to 5° C. in an ice bath, and a solution of diethyl propylmalonate (10.1 g, 0.05 mol) in THF (30 mL) was quickly dropped into it. After dropping, keep the reaction at 5°C-10°C for 1h. Chloromethylbenzyl ether (7.8 g, 0.0 mol) in THF (tetrahydrofuran, 20 mL) was added dropwise, and the temperature was controlled at 5°C to 10°C. After dropping, keep the reaction at 5°C-10°C for 1h. Sampling and testing showed that the reaction was complete. Add 10 mL of water to quench the reaction, add 30 mL of 1N hydrochloric acid, and extract and separate layers with 100 mL of EA (ethyl acrylate). The organic phase was successively washed with water, saturated sodium bicarbonate and saturated sodium chloride, dried over anhydrous sodium sulfate, filtered and spin-dried to obtain intermediate A colorless liquid 16g, ESI(M+1)=323.
[0043] Intermediate A (31g, 0.0963mol) was dis...
Embodiment 2
[0064] (1) Potassium tert-butoxide (1.2 g, 0.01 mol) was dissolved in 20 mL THF. The reaction solution was cooled to 5° C. in an ice bath, and a solution of diethyl propylmalonate (2.0 g, 0.01 mol) in THF (10 mL) was quickly dropped into it. After dropping, keep the reaction at 5°C-10°C for 1h. Chloromethyl methyl ether (0.8 g, 0.01 mol) in THF (tetrahydrofuran, 10 mL) was added dropwise, and the temperature was controlled at 5°C to 10°C. After dropping, keep the reaction at 5°C-10°C for 1h. Sampling and testing showed that the reaction was complete. Add 10mL of water to quench the reaction, add 10mL of 1N hydrochloric acid, extract with 20Ml*3EA (ethyl acetate), combine the organic phases, wash with water, saturated sodium bicarbonate, and saturated sodium chloride successively, dry over anhydrous sodium sulfate, filter and spin dry , to obtain intermediate A colorless liquid 2.5g, ESI(M+1)=247.
[0065] Intermediate A (2.5g, 0.01mol) was dissolved in 20mL of ethanol, and...
Embodiment 3
[0071] (1) Methyl valerate and methyl formate are transesterified to generate a formyl compound, then reduced by sodium borohydride to obtain a hydroxyl compound, then protected by TBDMS, and the methyl ester is hydrolyzed to obtain TBDMS-protected compound C (i.e. carboxylic acid Compound C); The reaction scheme is as follows:
[0072]
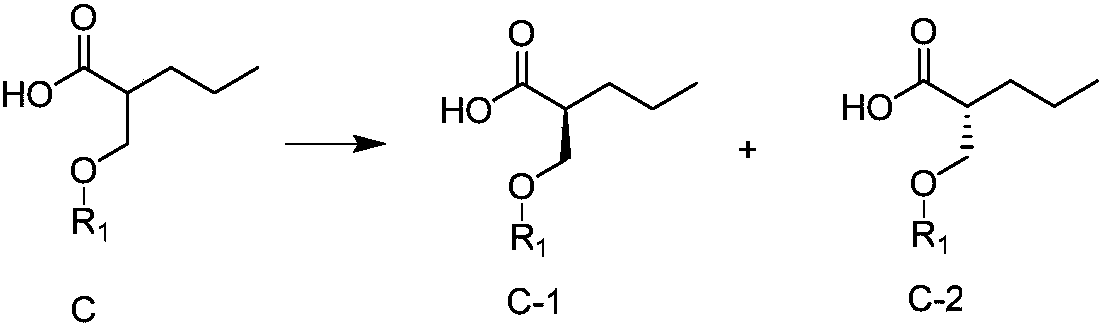
[0073] (2) Carrying out chiral resolution of carboxylic acid compound C in the same method as step (2) in Example 1 to obtain R-configured carboxylic acid compound C-1 and S-configured carboxylic acid compound C-2.
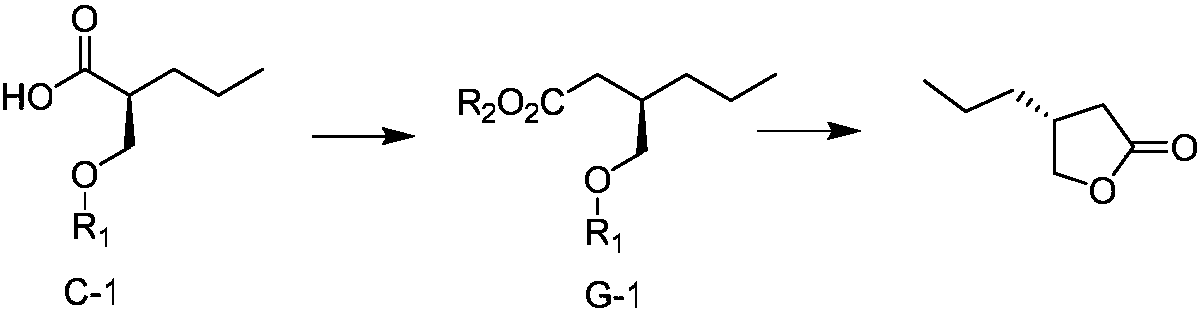
[0074] (3) R configuration carboxylic acid compound C-1 is operated and reacted according to conventional reaction conditions to generate alcohol intermediate D-1, and then reacted according to the following synthetic route:
[0075]
[0076] Specifically, alcohol intermediate D-1 (2.4g, 0.01mol) was dissolved in 20mL of DCM, triphenylphosphine (4.0g, 0.015mol, 1.5eq) was added, replaced with nitrogen, cooled in an ice bath...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com