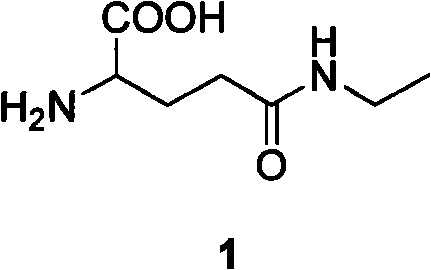
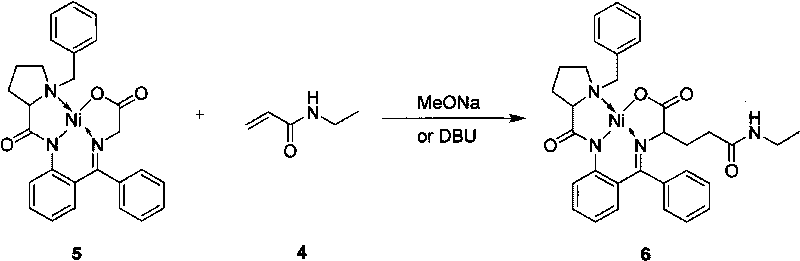
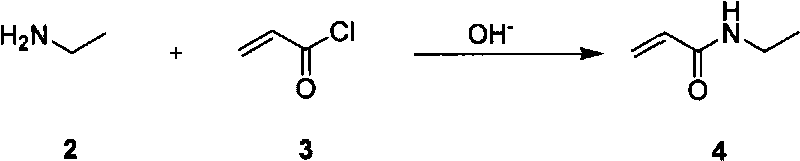Method for preparing L-theanine by chemical method
A theanine, chemical method technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of easy racemization of products, low yield, etc., and achieve the effect of good economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of N-benzylproline.
[0031] L-proline (0.30 mol) and potassium hydroxide (0.90 mol) were dissolved in 200 mL of isopropanol, under nitrogen protection, stirred, and heated to 40°C. Benzyl chloride (0.45mol) was slowly added dropwise, maintaining the temperature and stirring was continued for 6h. Neutralize to pH=6 with concentrated hydrochloric acid, add 80 mL of chloroform to the reaction mixture, and let stand overnight. Filter, wash the filter cake with chloroform until the washing liquid is colorless, combine the organic phases, evaporate the solvent, recrystallize the crude product with acetone, and dry it under vacuum at 60°C to obtain 57.9 g of N-benzylproline, with a yield of 94.0% , m.p.175~176℃; 1 H NMR (CDCl 3 , 300MHz) δ: 9.90(s, 1H), 7.46~7.28(m, 5H), 4.45, 4.17(AB, J=13.0Hz, 2H), 3.81(dd, J=8.8, 6.6Hz, 1H), 3.70 ~3.62(m, 1H), 2.91~2.82(m, 1H), 2.36~2.20(m, 2H), 2.06~1.87(m, 2H); HRMS(ESI) calcd for C 12 h 15 NNaO 2 [M+Na] + ...
Embodiment 2
[0032] Embodiment 2: chiral auxiliary agent---the preparation of 2-[N-(N'-benzyl-prolyl)amino]benzophenone.
[0033] Dissolve the N-benzylproline (0.20mol) obtained in Example 1 in 200mL of dichloromethane, stir at 0°C, add thionyl chloride (0.4mol) dropwise, continue stirring after dropping, add 2-amino Benzophenone (0.20mol / 50mL dichloromethane) solution, return to room temperature, continue the reaction for 10h, add saturated aqueous sodium carbonate solution to stop the reaction, adjust pH=7, separate the organic phase, dry over anhydrous sodium sulfate, filter, and evaporate the solvent , the crude product was recrystallized from absolute ethanol, and dried under vacuum at 60°C to obtain 65.4 g of 2-[N-(N'-benzyl-prolyl)amino]benzophenone, with a yield of 85.1%, m.p.100-101 ℃; 1 H NMR (DMSO-d 6 , 500MHz) δ: 11.03(s, 1H), 8.30(dd, J=8.3, 0.8Hz, 1H), 7.74~7.72(m, 2H), 7.69~7.65(m, 1H), 7.60~7.54(m, 3H), 7.46(dd, J=7.8, 1.5Hz, 1H), 7.31~7.29(m, 2H), 7.20~7.17(m, 1H), 7.14...
Embodiment 3
[0034] Example 3: Preparation of glycine Schiff base Ni(II) complex.
[0035] The chiral auxiliary agent BPB (0.005mol), nickel chloride hexahydrate (0.01mol) and glycine (0.025mol) obtained in Example 2 were dissolved in 17.5mL of methanol, under nitrogen protection, stirred, and heated to 40-50°C. Add potassium hydroxide (0.035mol / 7.5mL methanol) solution, stir at 55-65°C for 1h, cool to room temperature, add acetic acid (0.035mol) under stirring, pour the above mixture into 75mL of water, and a large amount of crystals are precipitated. It was filtered and the crystals were washed twice with water. The crude product was recrystallized with acetone and dried under vacuum at 60°C to obtain 2.4 g of a glycine Schiff base Ni(II) complex with a yield of 96.3%, m.p.219-220°C; 1 H NMR (CDCl 3 , 500MHz) δ: 8.31(d, J=8.7, 1H), 8.07(d, J=7.1Hz, 2H), 7.53~7.49(m, 3H), 7.43(t, J=7.5Hz, 2H), 7.31 (t, J=7.4Hz, 1H), 7.23~7.19(m, 1H), 7.10(d, J=7.1, 1H), 6.98(d, J=4.5Hz, 1H), 6.80(dd, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com