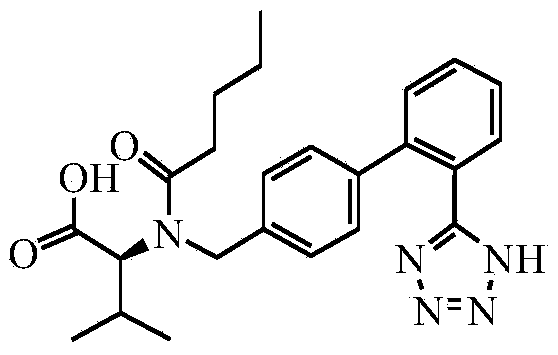
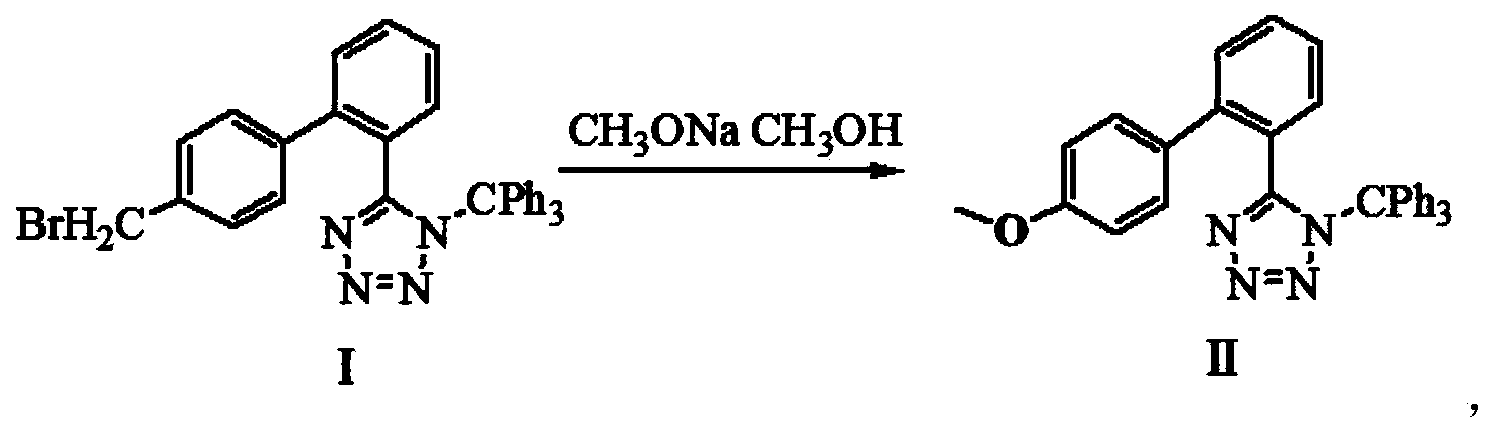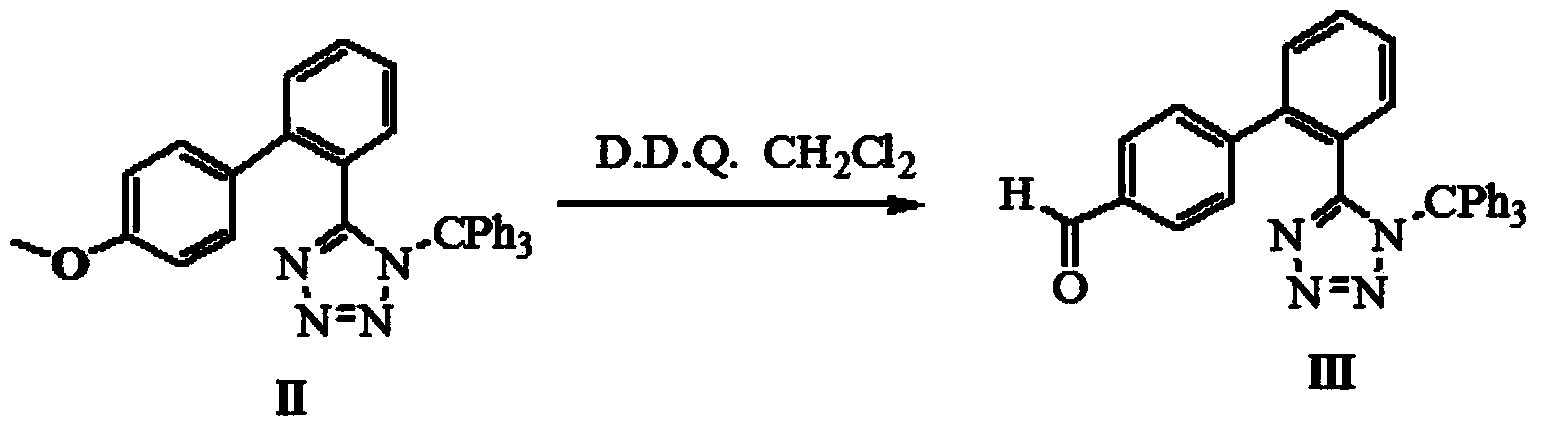Method for preparing valsartan
A technology of valsartan and compound, which is applied in the field of drug synthesis to achieve the effect of maintaining optical purity and avoiding the problem of product racemization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1. Preparation of Valsartan
[0044] Add 20mL of methanol, 5.56g of N-(trityl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium and 0.81g of sodium methoxide into a 100mL flask at room temperature, and react for 180 minutes at room temperature . After TLC showed that the reaction was complete, the reaction solution was filtered, and 25 mL of dichloromethane was added to the reaction solution, washed with saturated sodium bicarbonate until there were no bubbles, washed with water, separated, the organic phase was dried with anhydrous sodium sulfate, suction filtered, and reduced pressure Remove dichloromethane to obtain the product of the first step.
[0045] Dissolve the product from the previous step in dichloromethane, add 2.27 g of D.D.Q. and stir for 180 minutes at room temperature. After the reaction is complete, the reaction solution is filtered and spin-dried under reduced pressure to obtain the second-step product.
[0046] Under anhydrous conditions, put the produ...
Embodiment 2
[0053] 1. Preparation of Valsartan
[0054] Add 20mL of methanol, 5.56g of N-(trityl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium and 1.2g of sodium methoxide into a 100mL flask at room temperature, and react for 180 minutes at room temperature . After TLC showed that the reaction was complete, the reaction solution was filtered, and 25 mL of dichloromethane was added to the reaction solution, washed with saturated sodium bicarbonate until there were no bubbles, washed with water, separated, the organic phase was dried with anhydrous sodium sulfate, suction filtered, and reduced pressure Remove dichloromethane to obtain the product of the first step.
[0055] Dissolve the product from the previous step in dichloromethane, add 2.27 g of D.D.Q. and stir for 180 minutes at room temperature. After the reaction is complete, the reaction solution is filtered and spin-dried under reduced pressure to obtain the second-step product.
[0056] Under anhydrous conditions, put the produc...
Embodiment 3
[0063] 1. Preparation of Valsartan
[0064] Add 20mL of methanol, 5.56g of N-(trityl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium and 0.81g of sodium methoxide into a 100mL flask at room temperature, and react for 180 minutes at room temperature . After TLC showed that the reaction was complete, the reaction solution was filtered, and 25 mL of dichloromethane was added to the reaction solution, washed with saturated sodium bicarbonate until there were no bubbles, washed with water, separated, the organic phase was dried with anhydrous sodium sulfate, suction filtered, and reduced pressure Remove dichloromethane to obtain the product of the first step.
[0065] Dissolve the product from the previous step in dichloromethane, add 3.27 g of D.D.Q. and stir for 120 minutes at room temperature. After the reaction is complete, the reaction solution is filtered and spin-dried under reduced pressure to obtain the second-step product.
[0066] Under anhydrous conditions, put the produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com