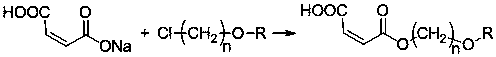Patents
Literature
45 results about "Ether formation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
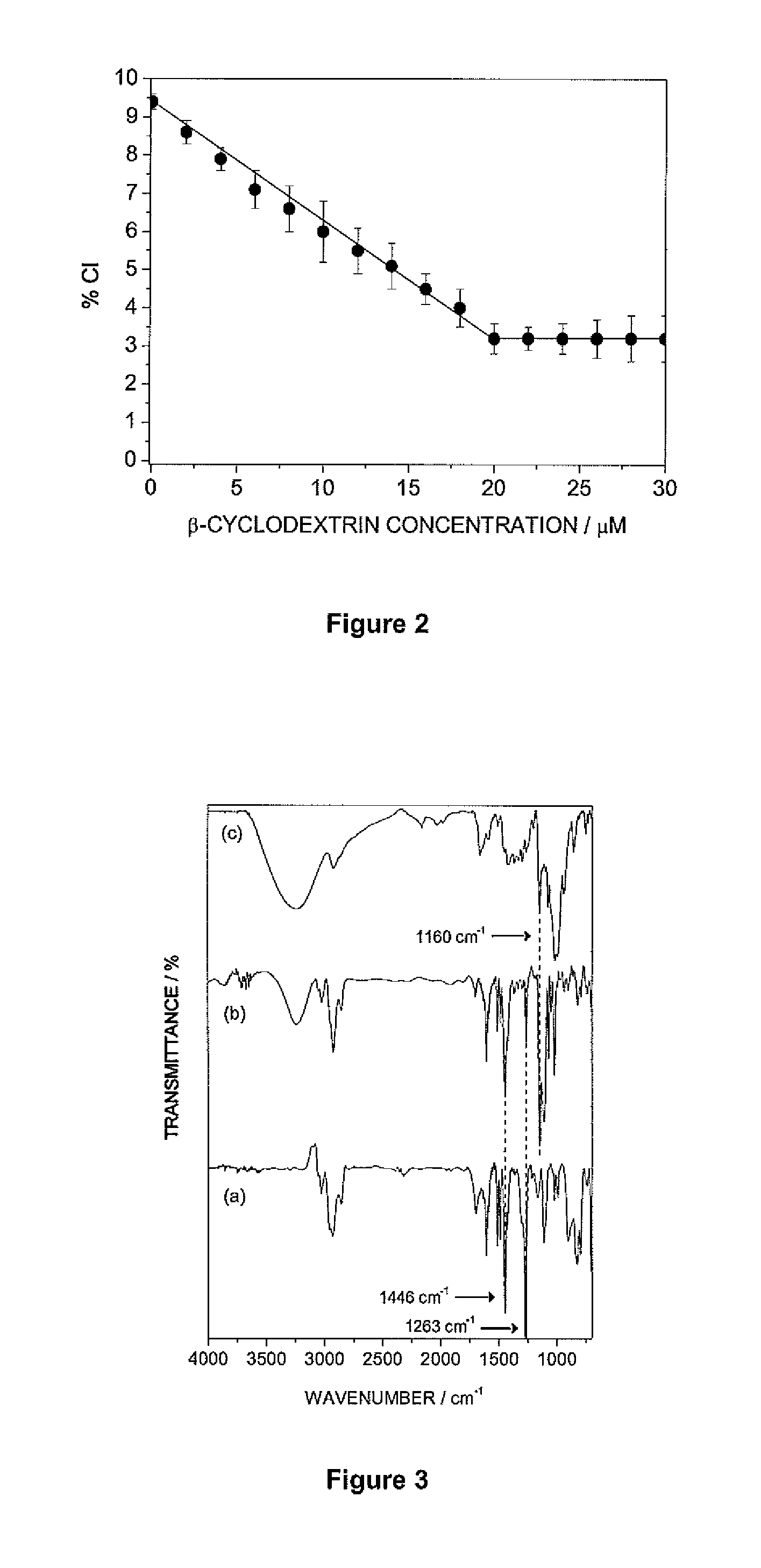
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction.
Preparation method and applications of oriented immobilized PEGA composite resin
ActiveCN104844710ADoes not affect conformationDoes not affect activityCarrier-bound/immobilised peptides4-nitrobenzyl alcoholTert-Butyloxycarbonyl protecting group
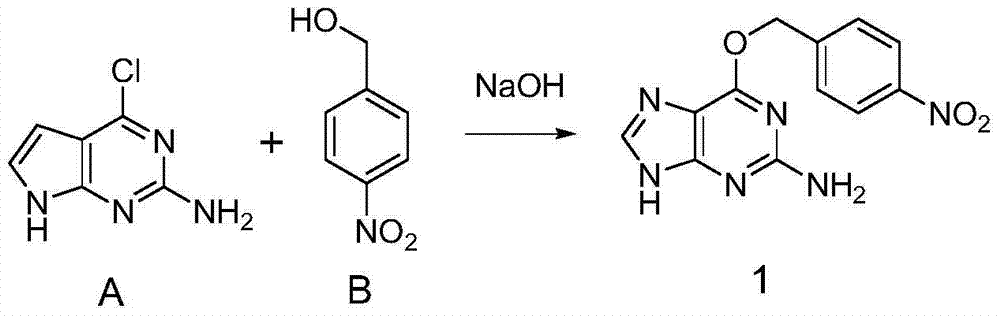
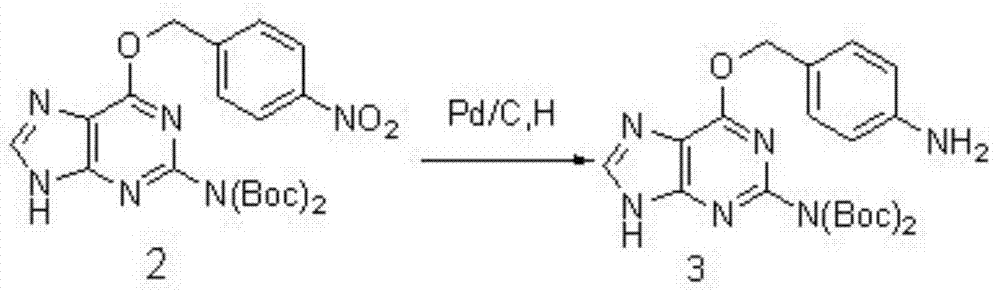
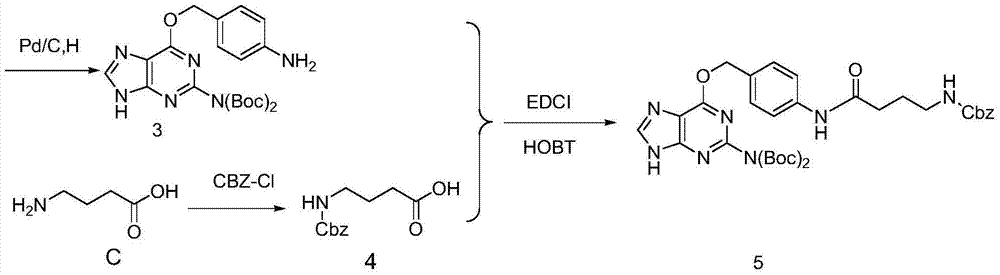
The invention discloses a preparation method and applications of an oriented immobilized PEGA composite resin. The preparation method comprises following steps: 2-amino-6-chloropurine and 4-nitrobenzyl alcohol are taken as raw materials for Williamson ether synthesis so as to obtain 4-nitryl-O6-benzylguanine; 4-nitryl- O6-benzylguanine is subjected to reduction reaction with protection of t-butyloxycarboryl; coupling reaction of an obtained compound with gamma-aminobutyric acid protected by carbobenzoxy chloride is carried out in the presence of ethyl dimethyl carbodiimide and 1-hydroxybenzotriazole; O<6>-benzylguanine derivative modified PEGA resin is obtained via reduction, separation and purification, reaction with PEGA, and deprotection; and transalkylation reaction of the O<6>-benzylguanine derivative modified PEGA resin with a protein containing MGMT label is carried out, so that oriented immobilization on PEGA resin surface via thioether covalent bonds is realized. Reaction of the preparation method is stable; the steps are simple; a stable uniform protein coating with uniform orientation can be formed on solid material surface by a prepared product; and the preparation method is used for preparing reagent kit with specific recognition effects.
Owner:NORTHWEST UNIV
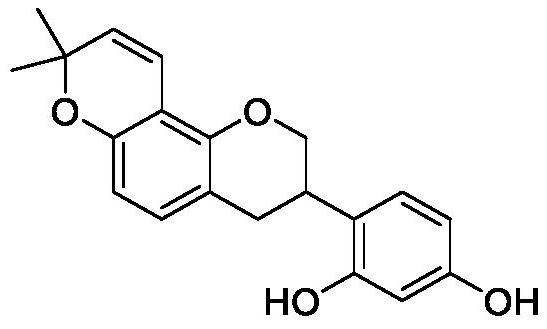
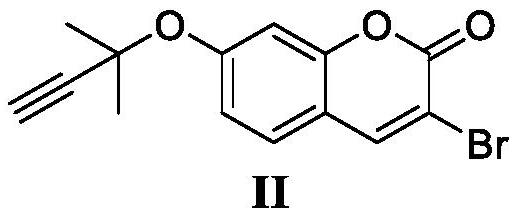
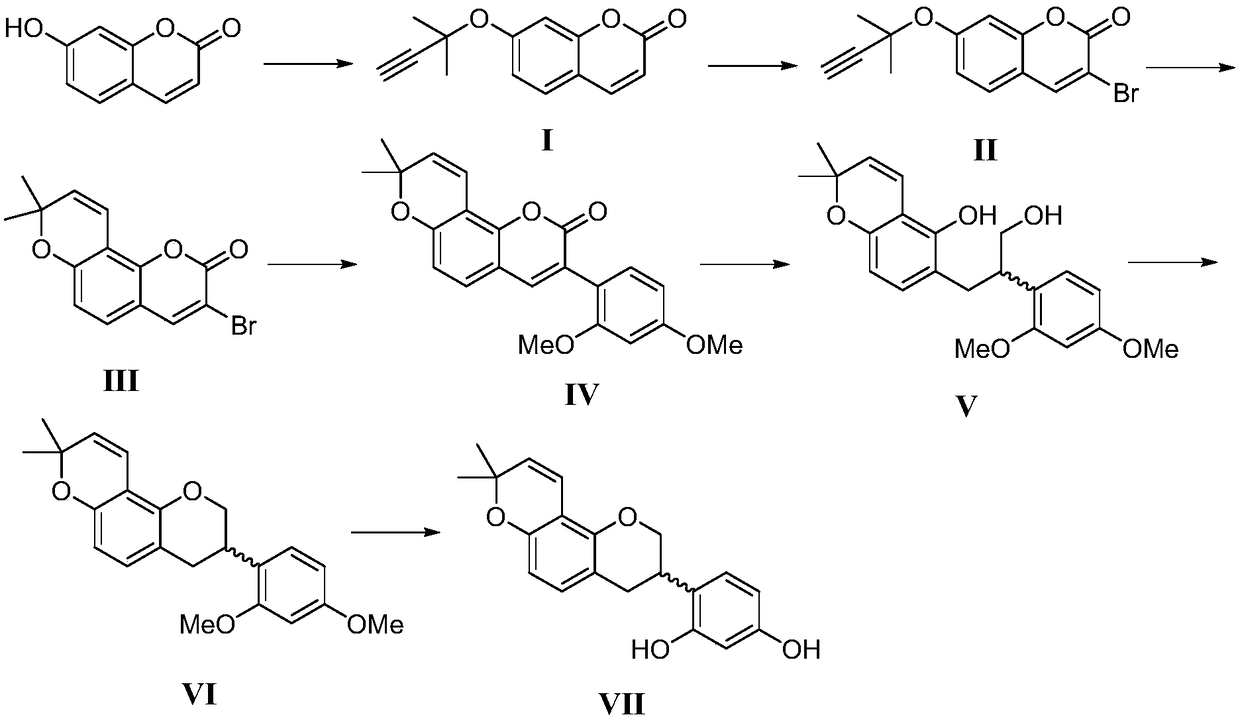
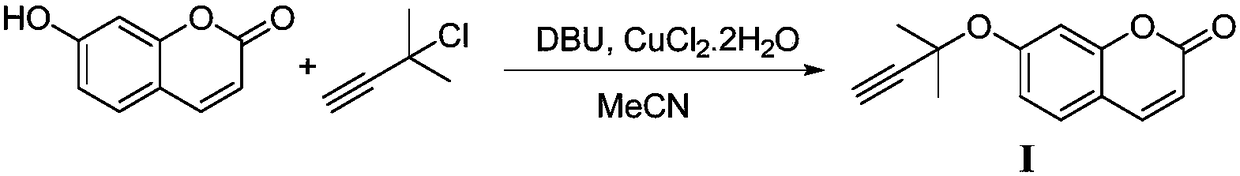
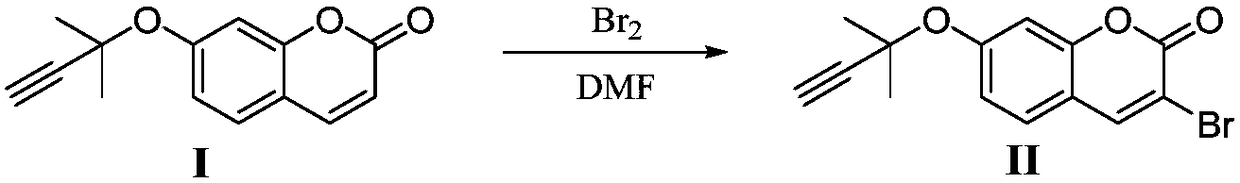
Synthesis method of glabridin
The invention discloses a synthesis method of glabridin. According to the method, cheap easy-to-obtain 7-hydroxycoumarin is used as a raw material to react with 2-methyl-3-butyne-1-alcohol for building alkyne ester; then, bromine atoms are introduced into alpha sites of obtained alpha-beta-unsaturated compounds; through seven-step reaction including rearrangement, Szuki coupling as core reactionsfor key intermediate (IV) building, dihydroxy compound obtaining through conjugated double bond reduction, Mitsunobu ether formation and methyl removal, glabridin is fast and efficiently synthesized at the total yield of 20 percent finally. Compared with the prior art, the synthesis method has the following advantages that the raw materials are cheap and can be easily obtained; the total route isshort; the total yield is high; the glabridin can be fast and efficiently synthesized.
Owner:SHAANXI NORMAL UNIV
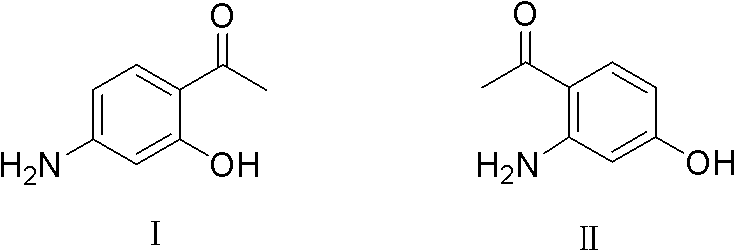
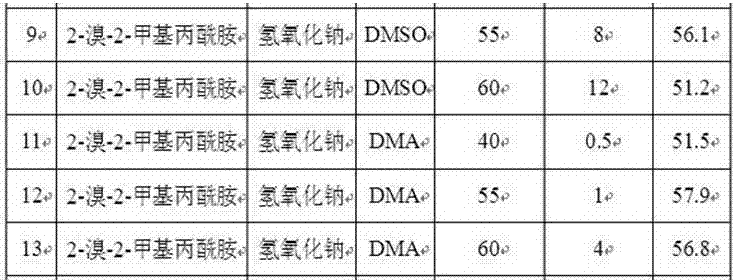
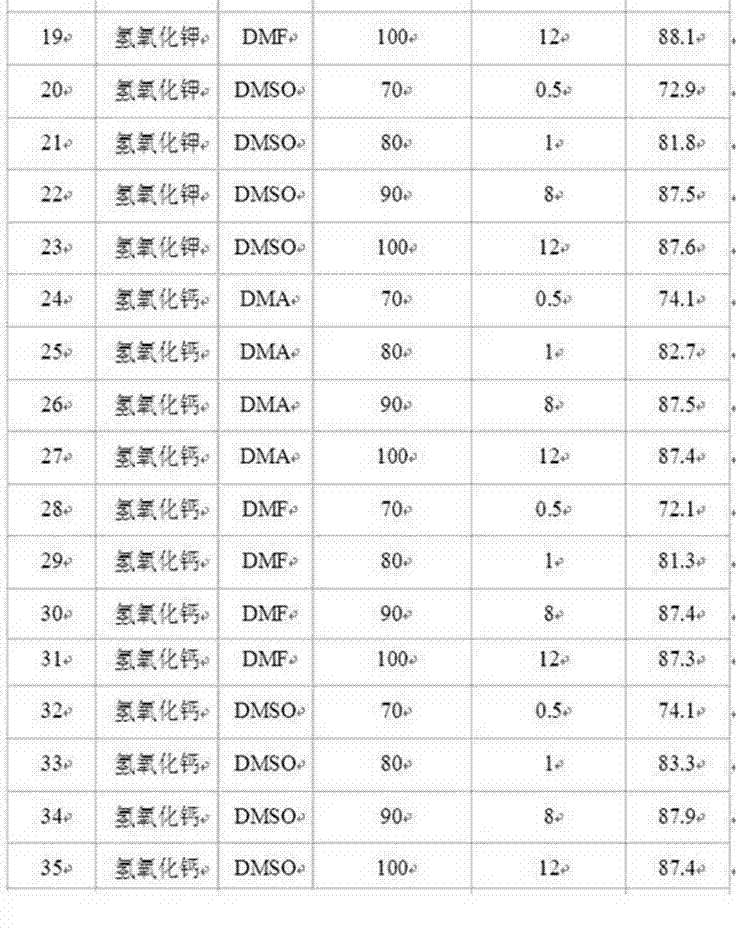
Synthesis method of 2-[(-4-chlorophenyl)(4-piperidinyl-oxy)methyl]pyridine having single optical isomer
InactiveCN104031029ADon't need protectionReduce generationOrganic chemistryChlorobenzeneCombinatorial chemistry
A preparation method of a compound of formula I is characterized in that the compound of formula I is obtained through one step direct condensation ether formation on a raw material of formula II and a raw material of formula III. The protection of reactive hydrogen on the nitrogen atom of the compound of the formula III is not needed in the invention, and a sulfonic acid compound is adopted as a reaction assistant, so the side reaction is reduced, reaction steps are simplified, and a satisfactory product yield can be realized. The compound of formula I having a single optical isomer can be obtained when the raw material of formula II having different isomers is selected.
Owner:CHONGQING HUAPONT PHARMA
Product
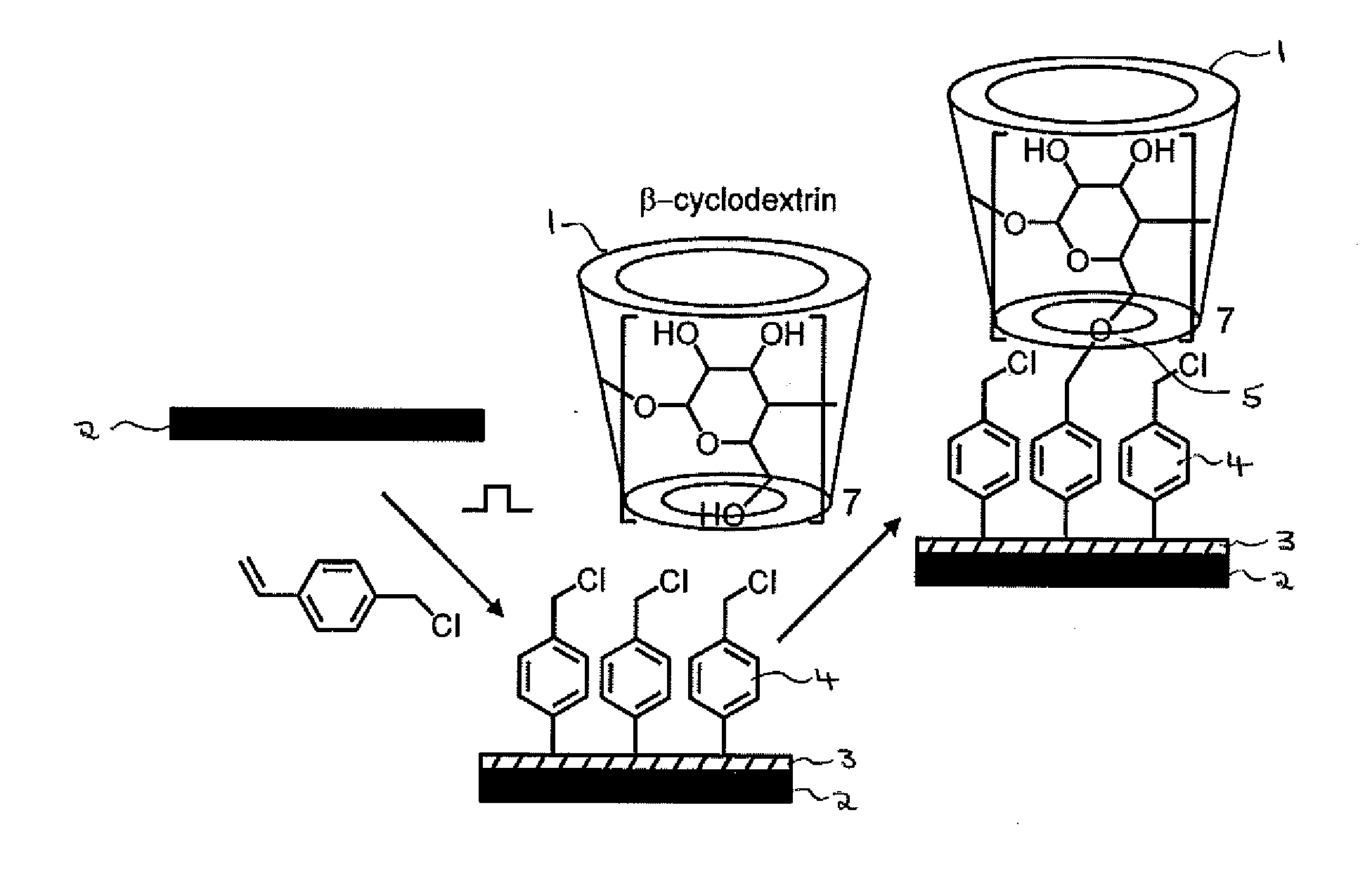
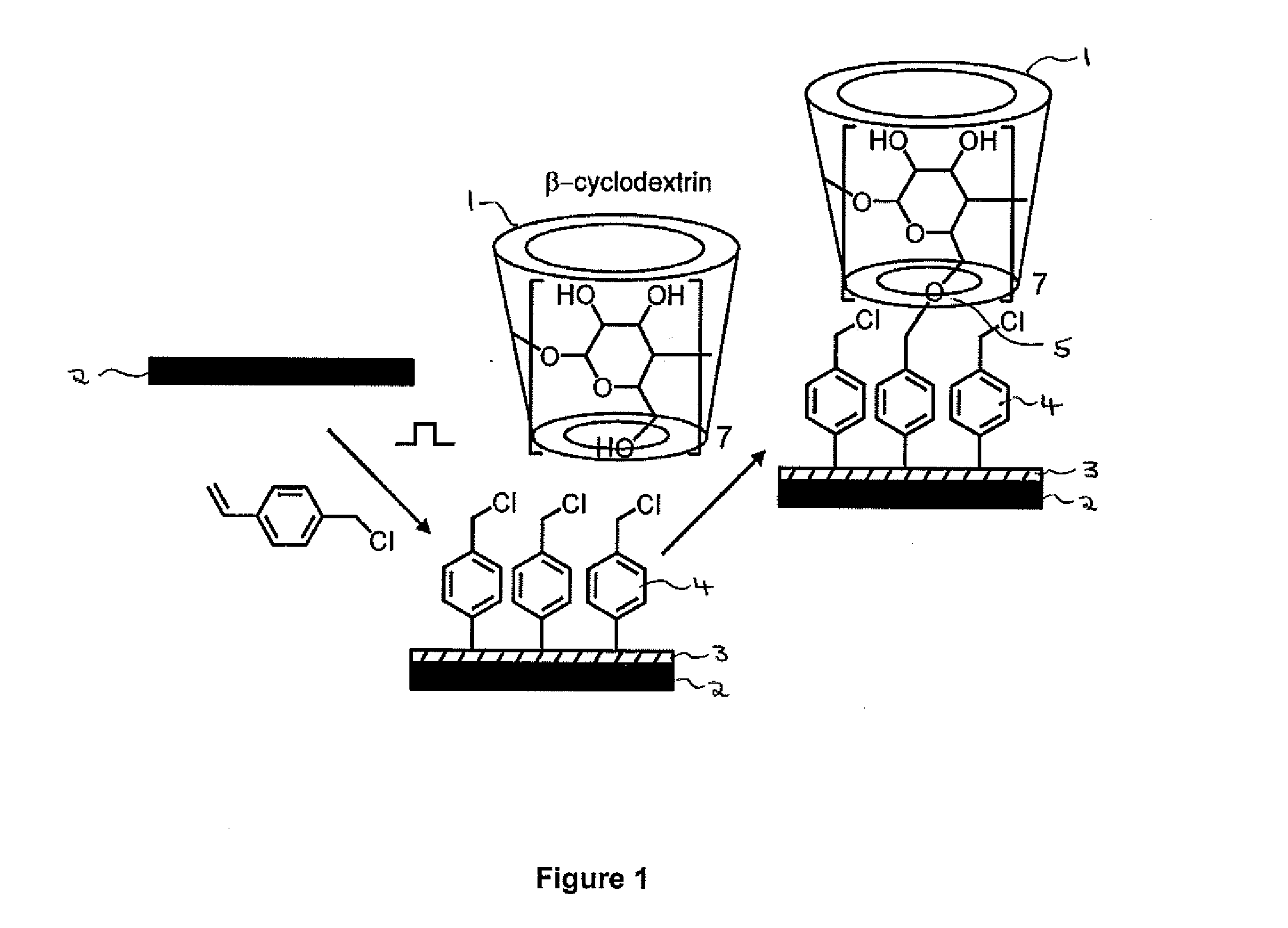
InactiveUS20140315780A1Easy loadingFacilitated releaseCosmetic preparationsToilet preparationsChemical LinkageCyclodextrin
A delivery system for an active substance, comprising a substrate on which the substance is loaded for subsequent release, wherein: (i) the substrate has been at least partially coated with a polymer using plasma deposition (preferably pulsed plasma deposition); (ii) the active substance is present as a guest molecule within a cyclodextrin inclusion complex; and (iii) the inclusion complex is bound to the polymer through a chemical linkage formed between a hydroxyl group on the cyclodextrin and a functional group on the polymer. The system may be used to control the release of an active substance such as a perfume. Also provided are methods for preparing (a) the delivery system and (b) a functionalised substrate for use as part of the system, in which the polymer is suitably reacted with a cyclodextrin using an S N 2 nucleophilic substitution reaction, in particular a Williamson ether synthesis reaction.
Owner:SURFACE INNOVATIONS LTD
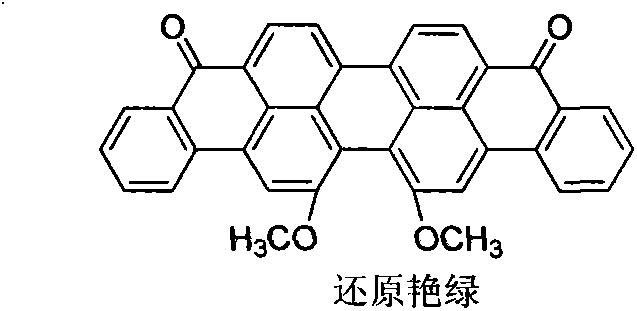
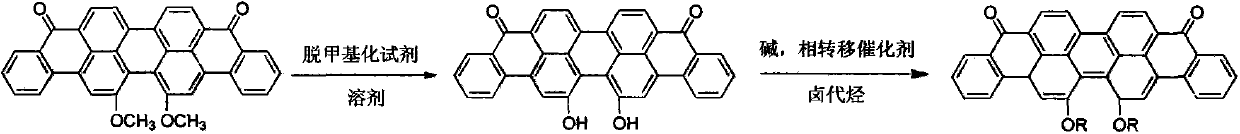
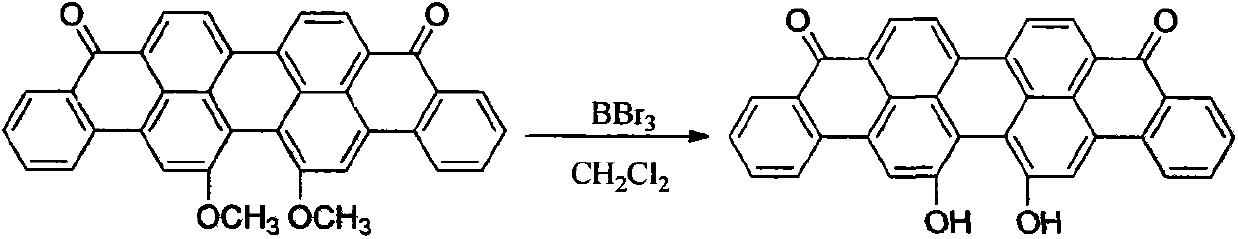
Method for preparing 16,17-dialkoxyviolanthrone derivatives
ActiveCN103804163AHigh yieldMild reaction conditionsOrganic compound preparationCarbonyl compound preparationMedicinal chemistryWilliamson ether synthesis
The invention discloses a method for preparing 16,17-dialkoxyviolanthrone derivatives. A readily available 16,17-dimethoxyviolanthrone dye is used as an initial raw material, a 16,17-dihydroxyviolanthrone intermediate is prepared through demethylation, and the corresponding 16,17-dialkoxyviolanthrone derivatives are synthesized through Williamson ether synthesis reaction. The method has the characteristics of short whole reaction route, high yield, mild reaction conditions and low cost.
Owner:BEIJING UNIV OF CHEM TECH
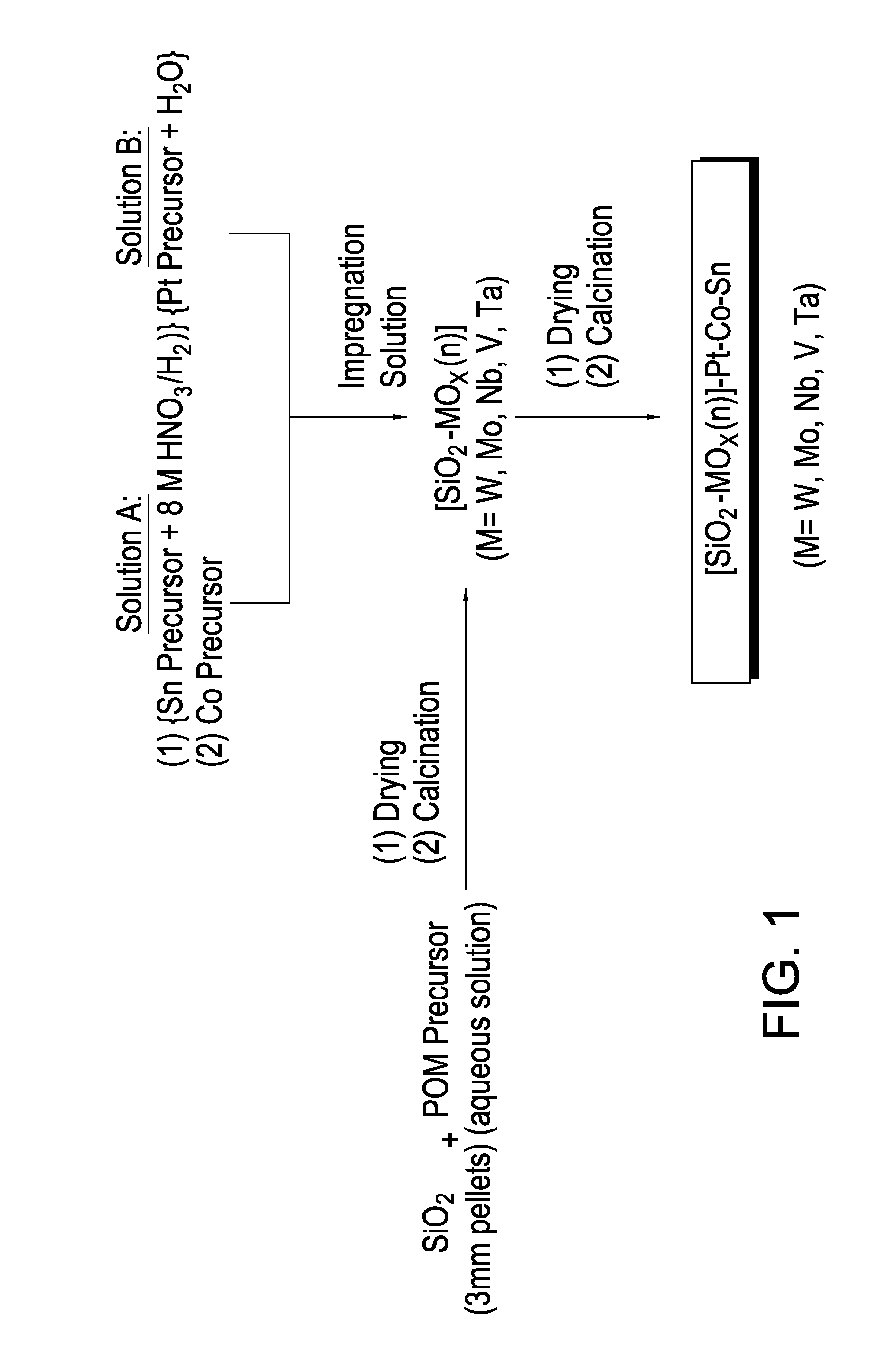
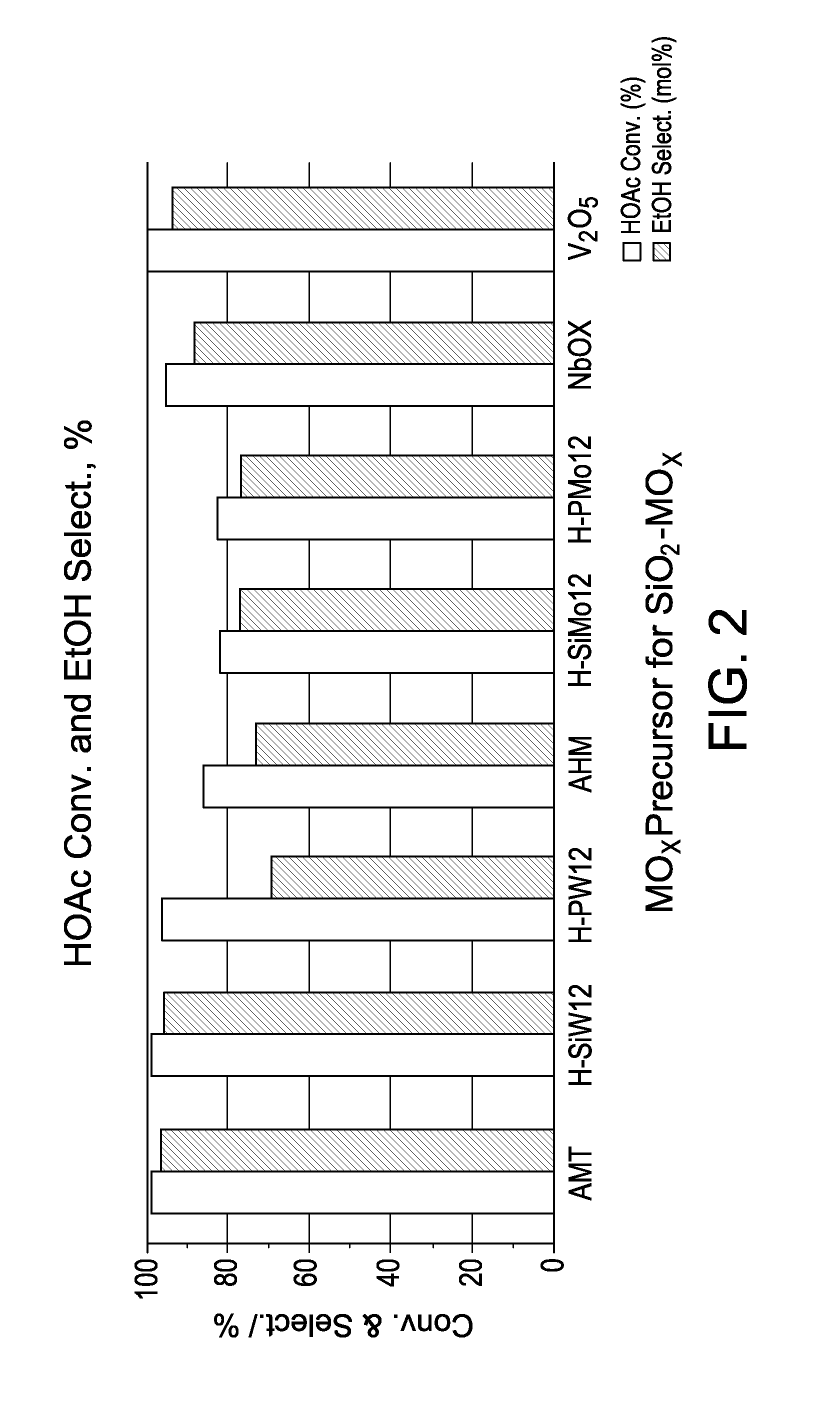
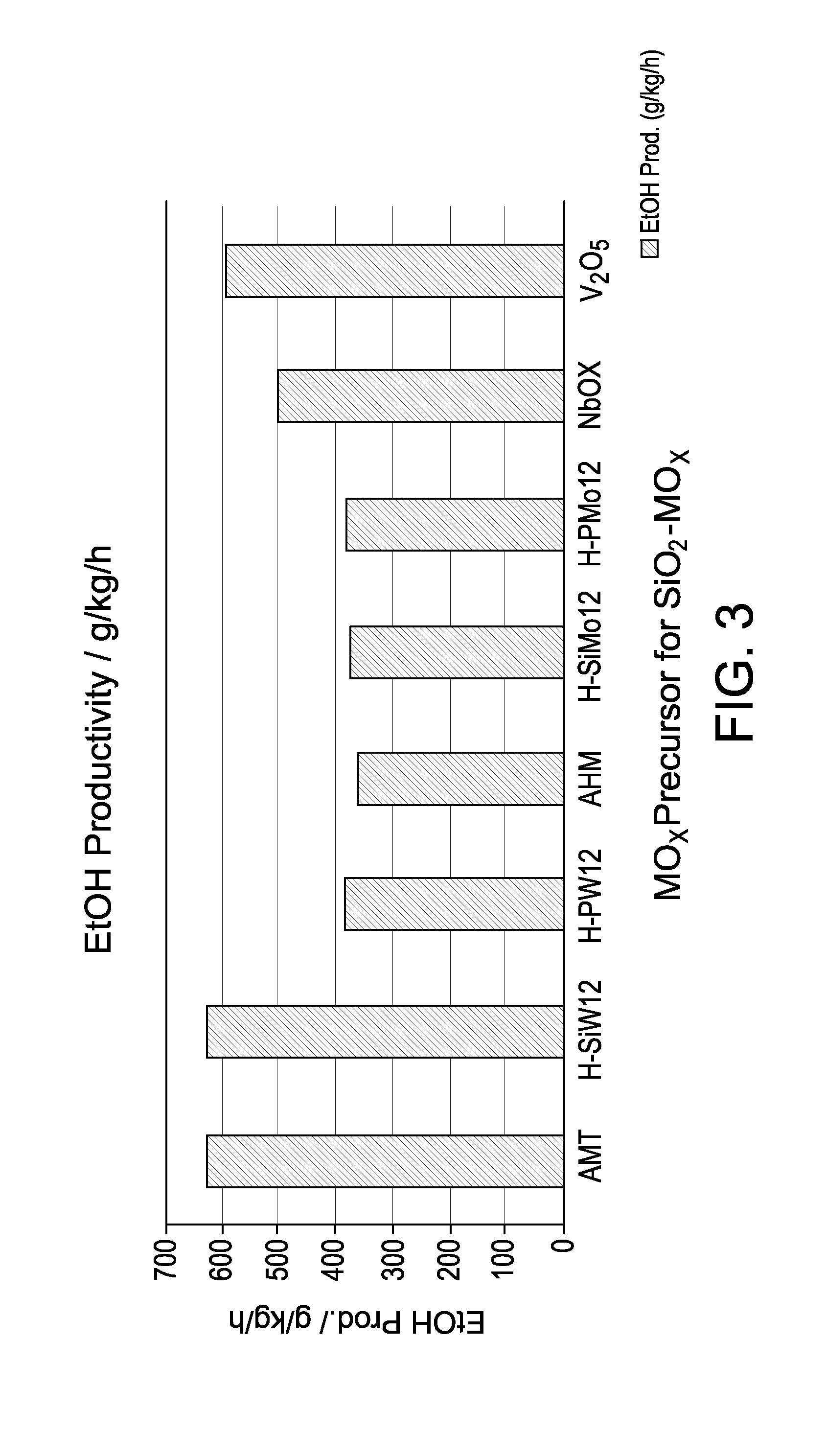
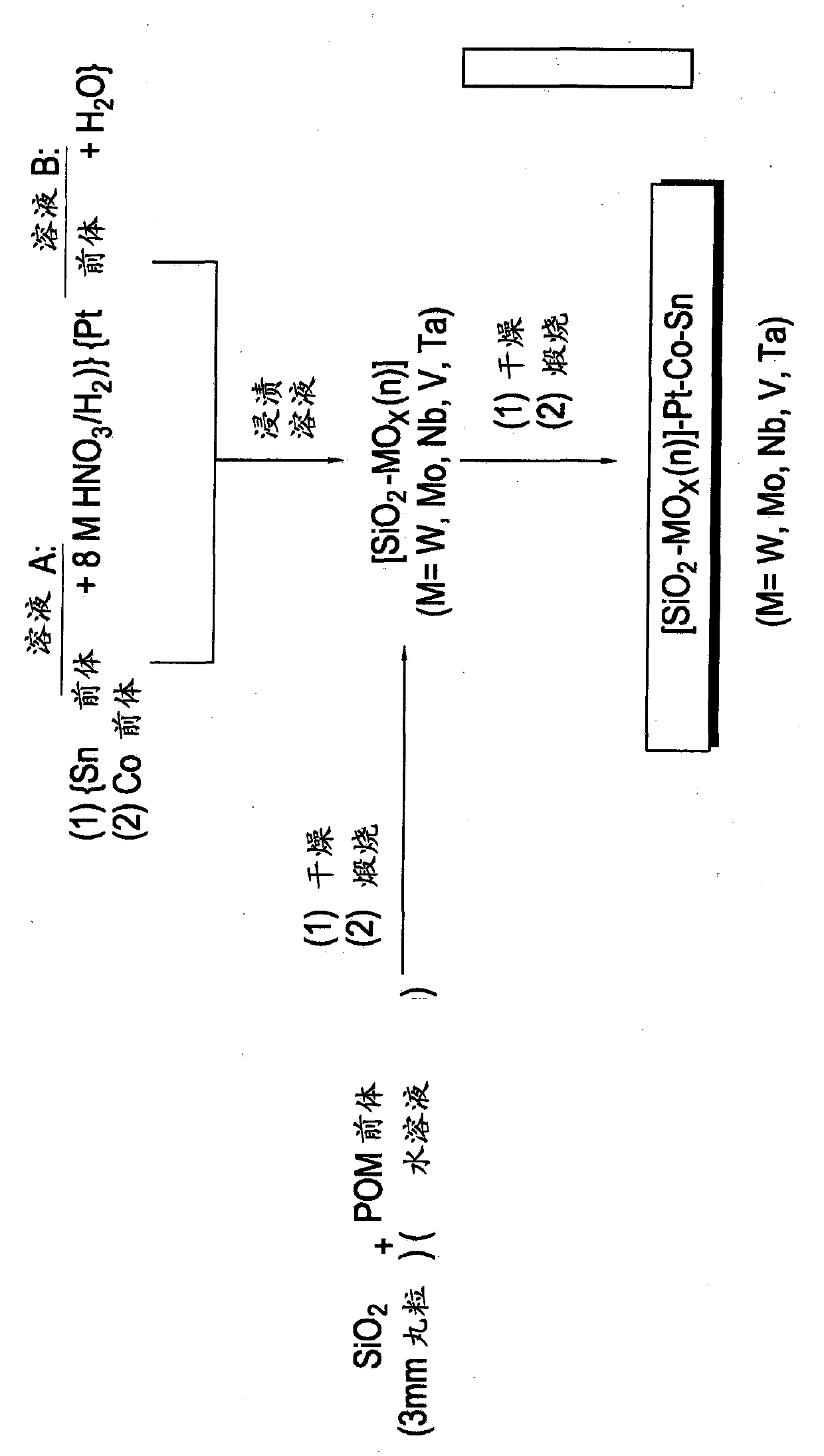
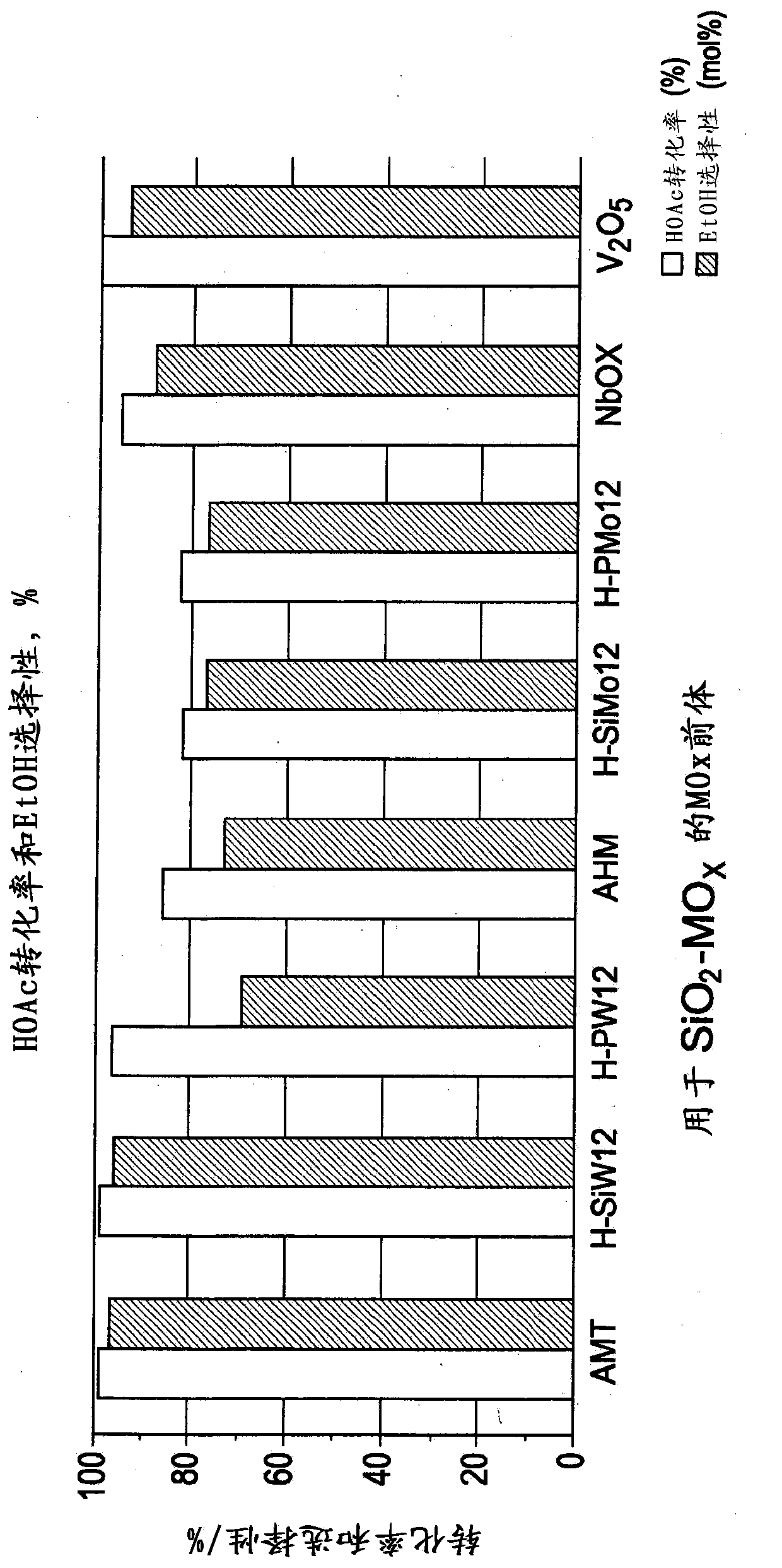
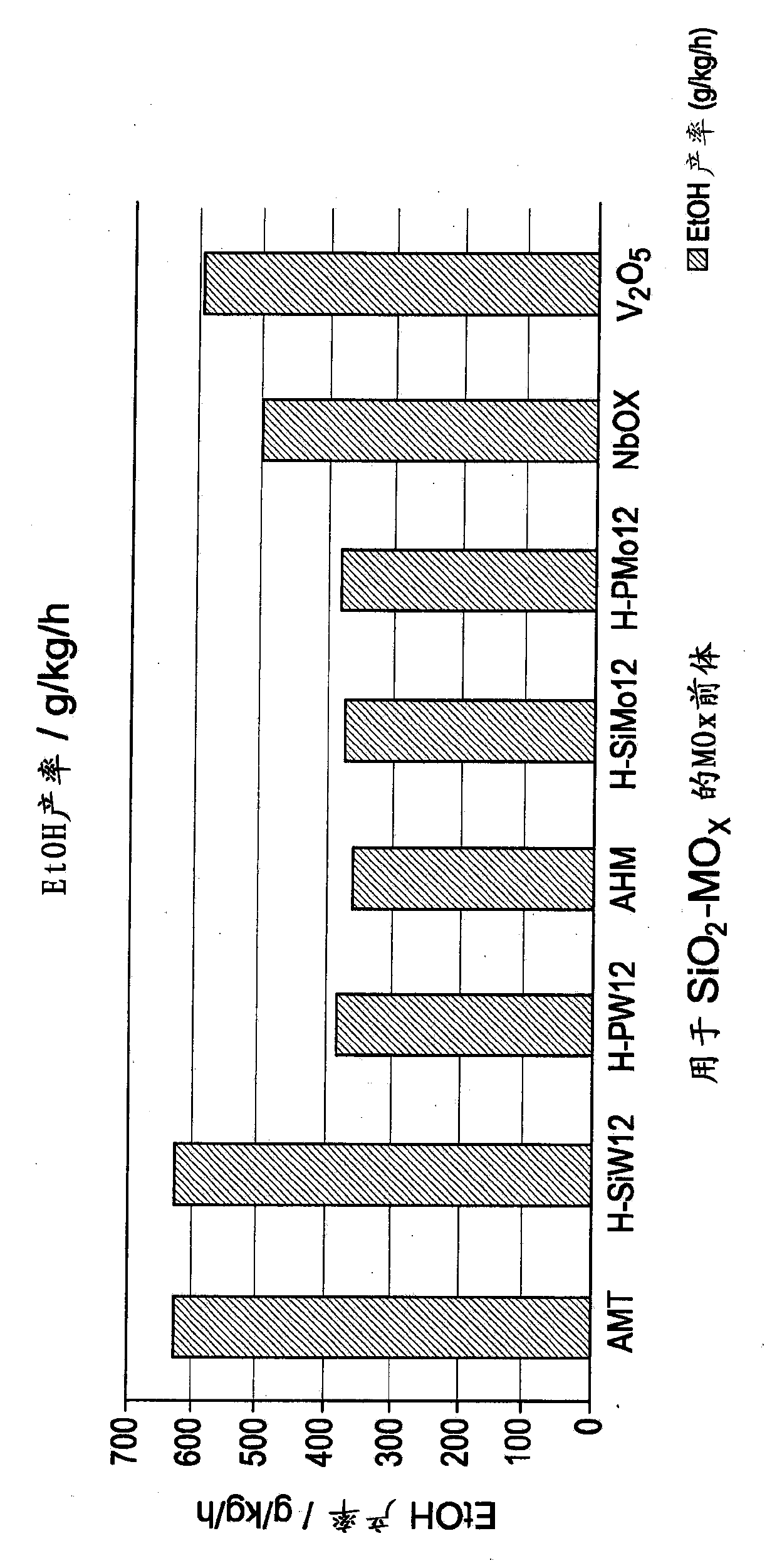
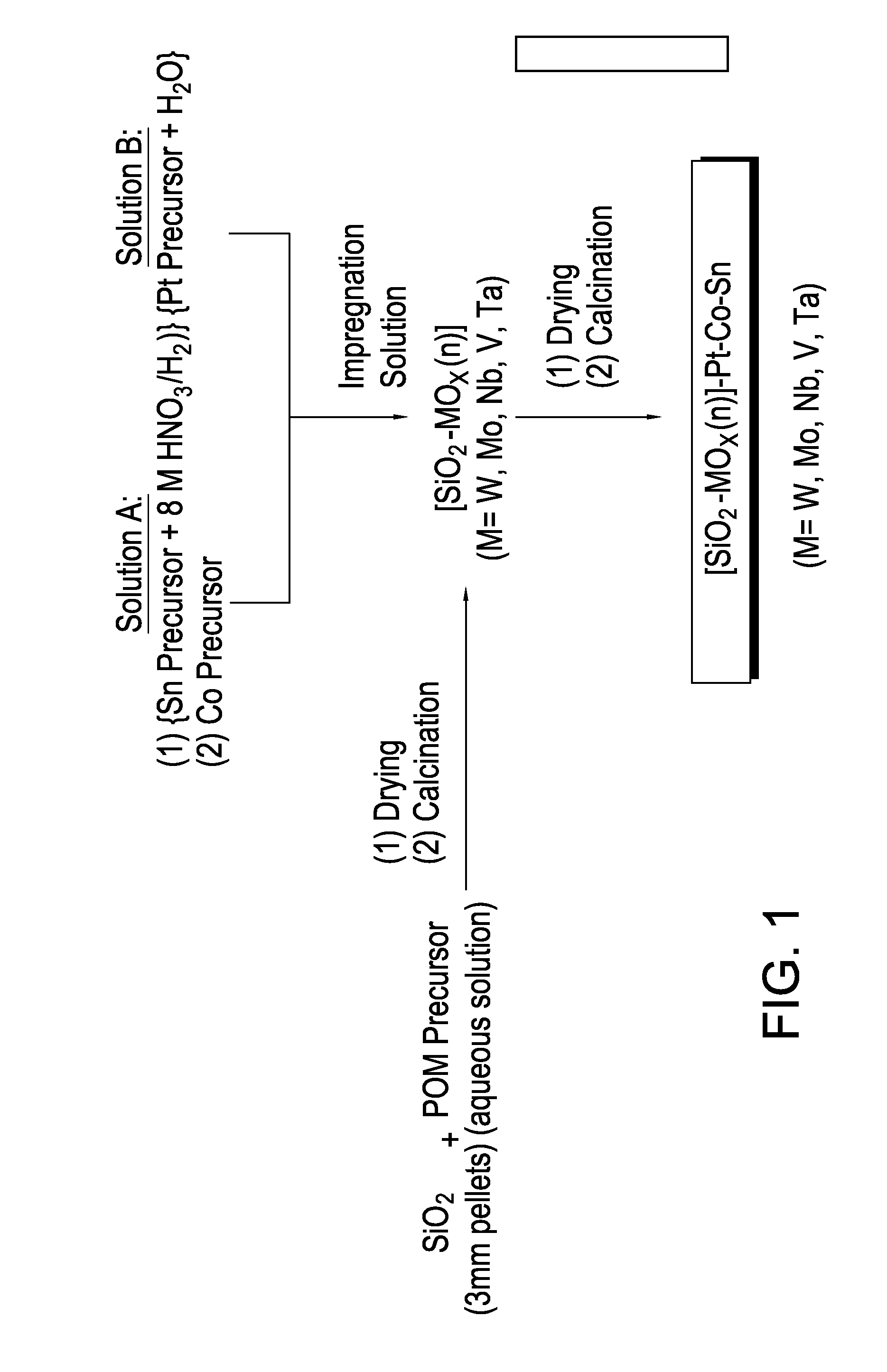
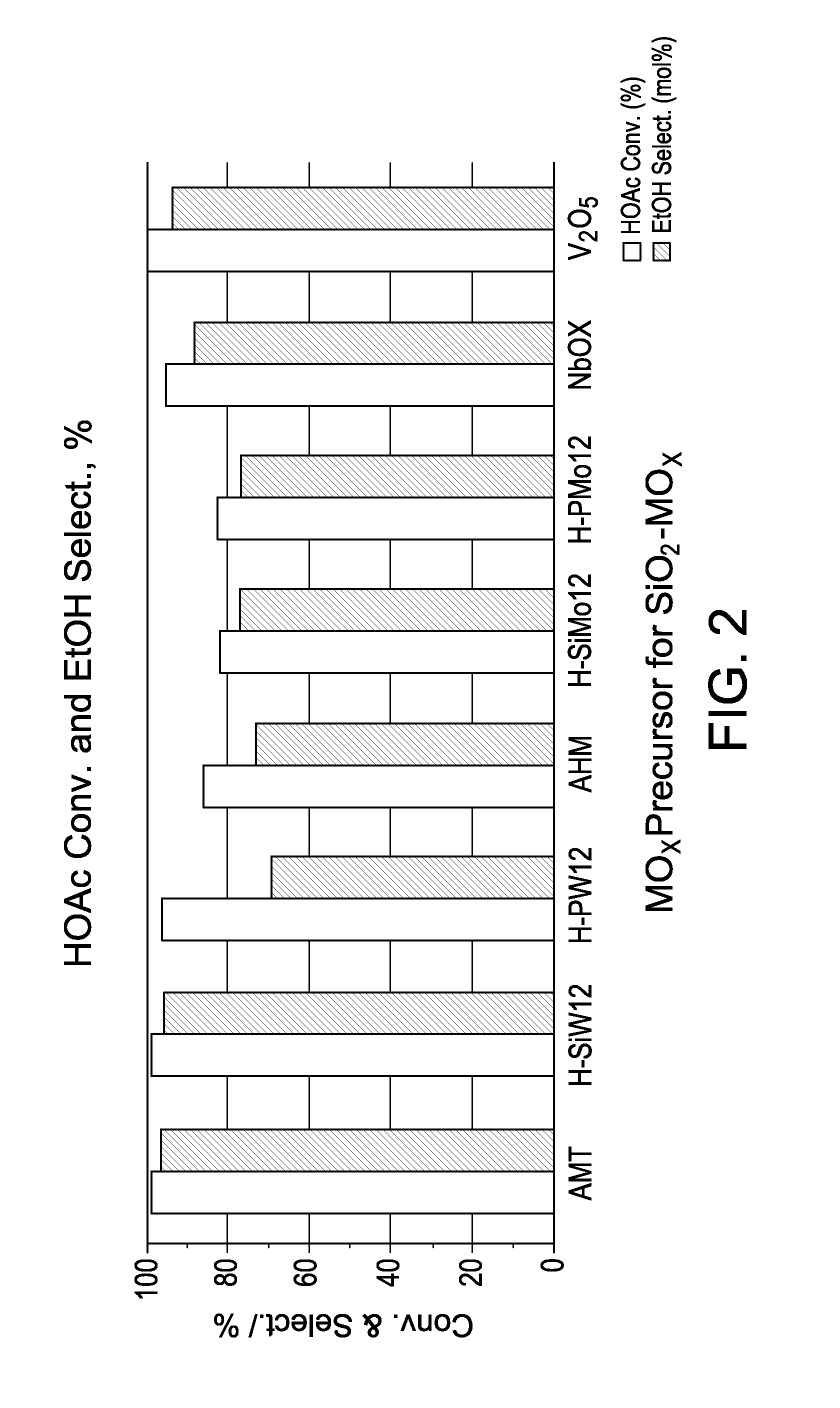
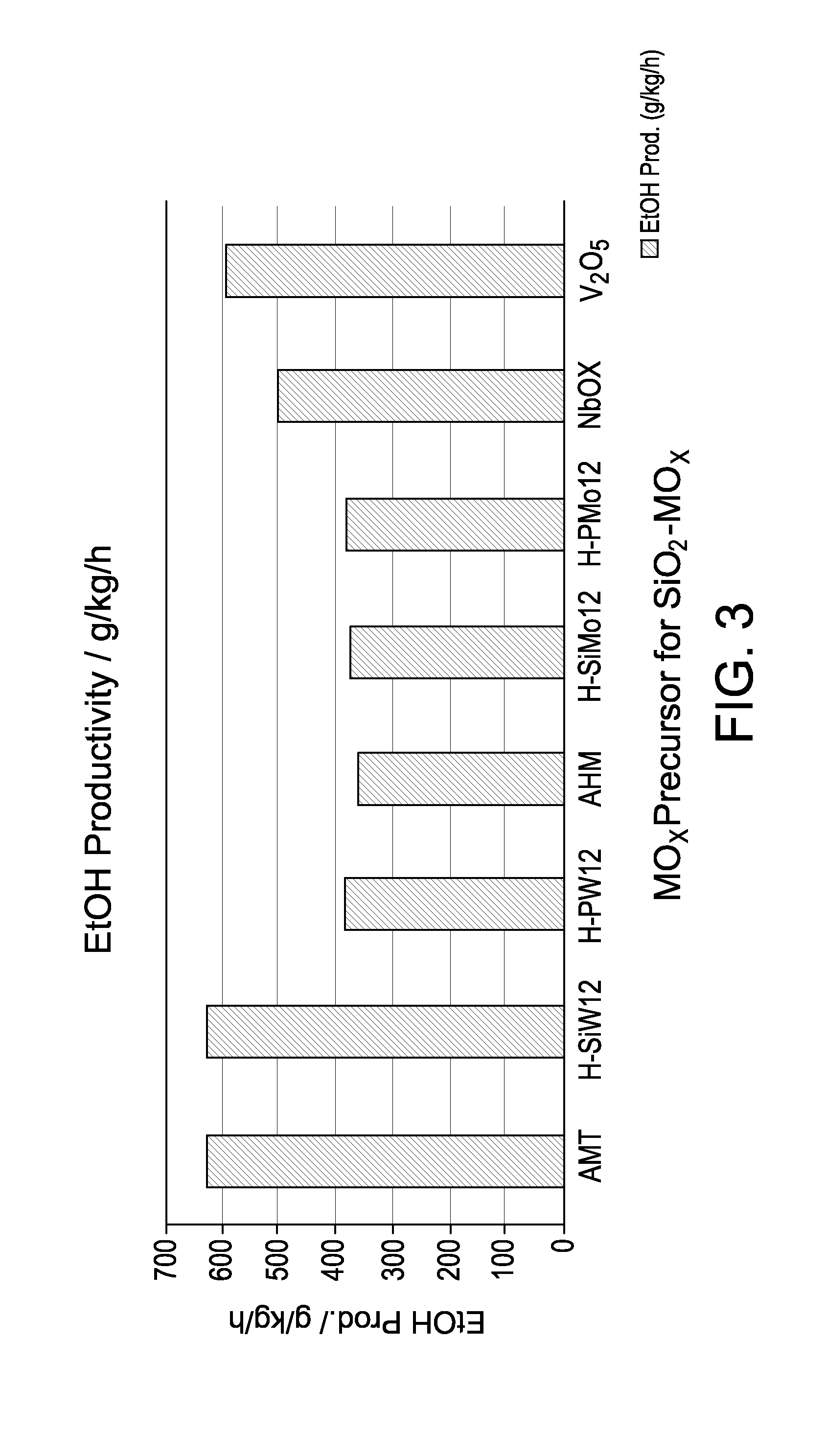
Hydrogenation catalysts prepared from polyoxometalate precursors and process for using same to produce ethanol while minimizing diethyl ether formation
InactiveCN103874545AOrganic compound preparationHydroxy compound preparationPtru catalystDiethyl ether
The present invention relates to hydrogenation catalysts prepared from polyoxometalate precursors. The polyoxometalate precursors introduce a support modifier to the catalyst. The catalysts are used for hydrogenating alkanoic acids and / or esters thereof to alcohols with relatively low ether formation, preferably with conversion of the ester coproduct. The catalyst may also comprise one or more active metals.
Owner:CELANESE INT CORP
Hydrogenation Catalysts Prepared from Polyoxometalate Precursors and Process for Using Same to Produce Ethanol While Minimizing Diethyl Ether Formation
InactiveUS20130245337A1Organic compound preparationHeterogenous catalyst chemical elementsAlcoholDiethyl ether
The present invention relates to hydrogenation catalysts prepared from polyoxometalate precursors. The polyoxometalate precursors introduce a support modifier to the catalyst. The catalysts are used for hydrogenating alkanoic acids and / or esters thereof to alcohols with relatively low ether formation, preferably with conversion of the ester coproduct. The catalyst may also comprise one or more active metals.
Owner:CELANESE INT CORP
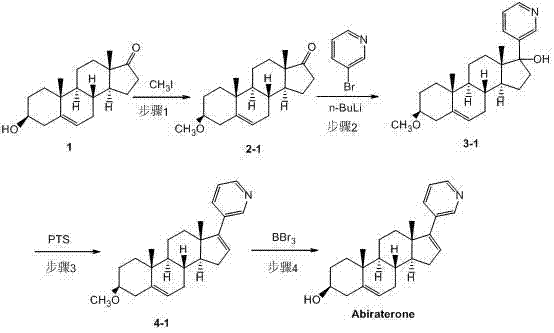
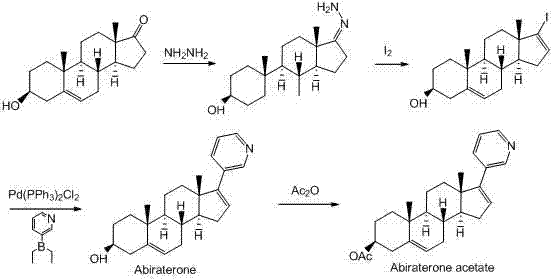
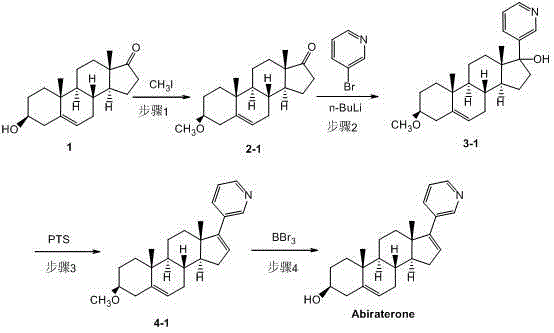
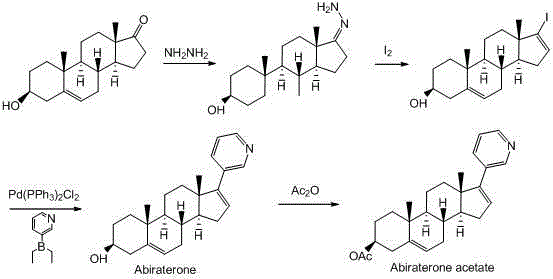
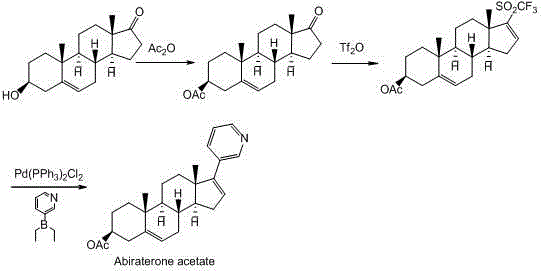
Synthesis method of abiraterone
ActiveCN103360458ALow priceEasy to getSteroidsBulk chemical productionOrganic solventGrignard reagent
The invention relates to a synthesis method of abiraterone. The synthesis method comprises the following steps of with dehydroepiandrosterone as a raw material, carrying out ether formation protection on hydroxyl by using a protecting group to obtain a compound; next, subjecting the compound, 3-bromopyridine and a Grignard reagent to reaction to obtain 3 beta-substituted oxo-17-(3-pyridyl)-androst-5-ene-17-ol; and heating the 3 beta-substituted oxo-17-(3-pyridyl)-androst-5-ene-17-ol, acid or a dehydrant and the like in an organic solvent, dehydrating, and then, removing the protecting group of the hydroxyl to obtain the abiraterone. The method provided by the invention has the advantages of simplicity and easiness in operation, adoption of low-toxicity raw materials and particular suitability for industrial production.
Owner:BRIGHTGENE PHARMA
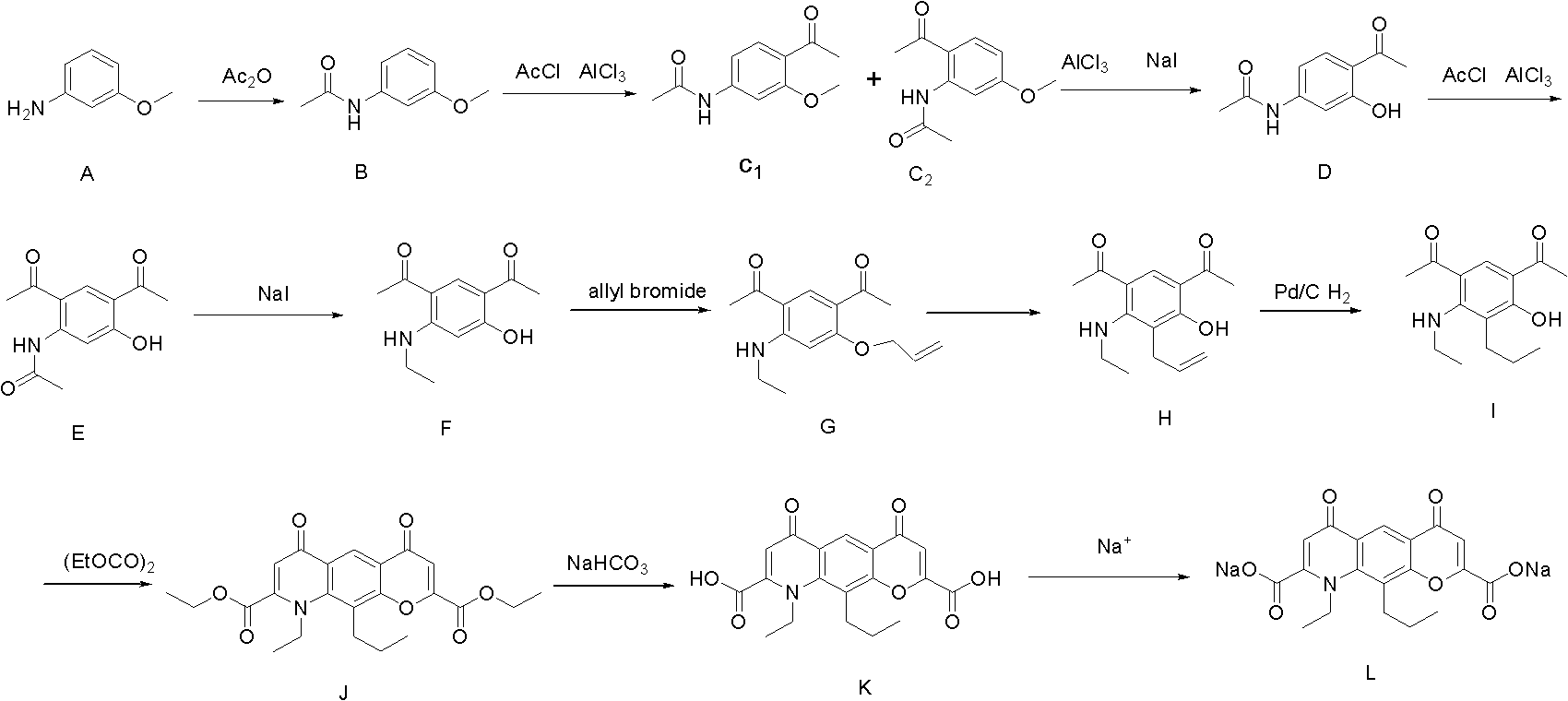
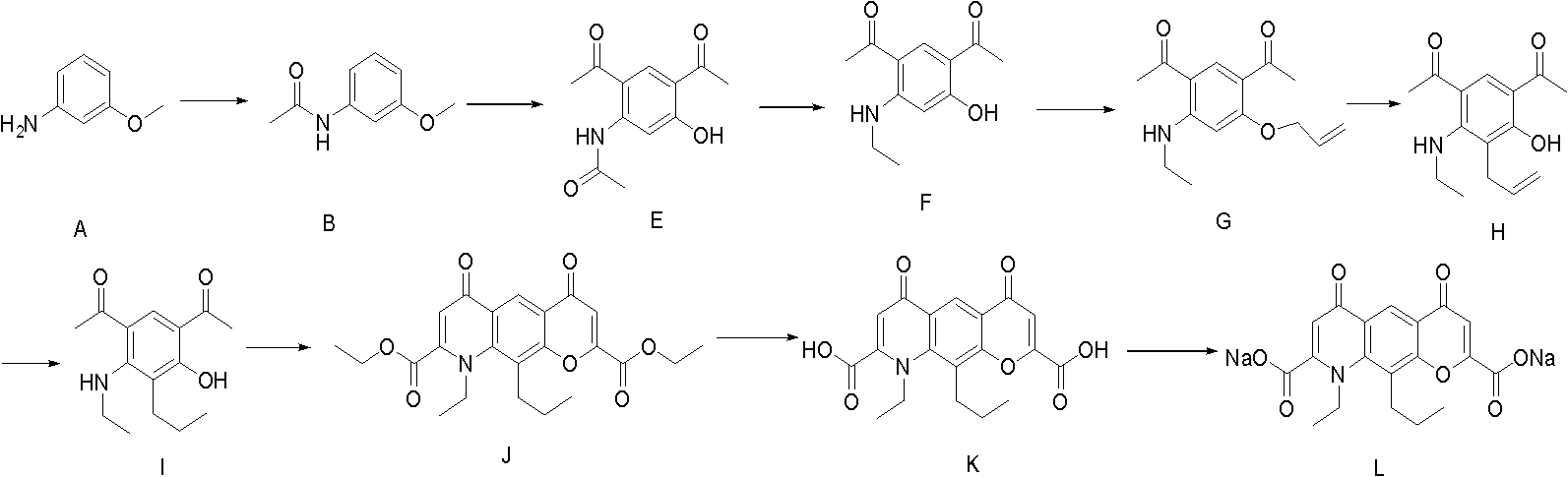
Preparation method of Nedocromil sodium
InactiveCN102516252AHigh synthetic yieldReduce manufacturing costOrganic compound preparationCarboxylic acid amides preparationNedocromil SodiumQuinoline
The invention discloses a preparation method of 9-ethyl-6,9-dihydro-4,6-dioxo-10-propyl-4H-pyrano[3,2-g] quinoline-2,8-sodium dicarboxylic acid (Nedocromil sodium). A raw material of m-anisidine (a compound A ) is treated with a condensation reaction to generate a compound B; the compound B is treated with a first Friedel-Crafts acylation to obtain a compound C1 and a compound C2; the compound C1 and the compound C2 are treated with demethylation, and isomer C2 is separated out to obtain a compound D; the compound D is treated with a second Friedel-Crafts acylation to obtain a diacetyl compound E; and the compound E is treated with a substitution reaction, ether formation, rearrangement, reduction, ring formation, hydrolysis and salt forming reaction to obtain the Nedocromil sodium (a compound L). The method of the invention increase synthesis yield of Nedocromil sodium.
Owner:SICHUAN DIHON MEDICAL DEV +1
Watermelon ketone preparation method
InactiveCN108164499AAvoid interferenceAvoid it happening againOrganic chemistrySodium bicarbonateKetone
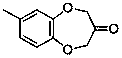
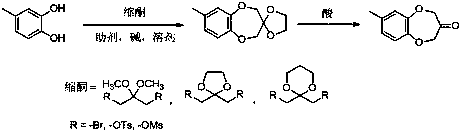
The invention discloses a watermelon ketone preparation method which comprises the following steps: (1) under an alkali condition and in the presence of aids, heating 4-methyl-orthodioxybenzene and alcohol-protected ketalized 1,3-bisubstited acetone to 30-120 DEG C in a solvent, and performing a Williamson ether synthesis reaction so as to generate a corresponding ketal intermittent, wherein reaction materials are fed in the following sequence: firstly, adding the solvent, an alkali and aids, uniformly stirring, heating to a set temperature, slowly dropping 4-methyl-orthodioxybenzene and alcohol-protected ketalized 1,3-bisubstited acetone, and performing a reaction for 2-12 hours after dropping is competed; (2) performing a backflow reaction on the ketal intermittent obtained in the step (1) for a certain time under an acid condition, pouring into water to implement hydrolysis, extracting with ethyl ether, washing an organic phase with a sodium bicarbonate solution, drying sodium sulfate, evaporating off the solvent, and performing recrystallization, thereby obtaining watermelon ketone. The method disclosed by the invention is easy to control, simple to operate, good in environmentprotection, relatively high in yield and beneficial to industrialization.
Owner:王成宇
Photo-responsive quaternary cationic surfactant capable of forming wormlike micelle and synthetic method thereof
ActiveCN106902702ASynthetic conditions are mildSimple purification methodOrganic chemistryTransportation and packagingCritical micelle concentrationEthylene Dibromide
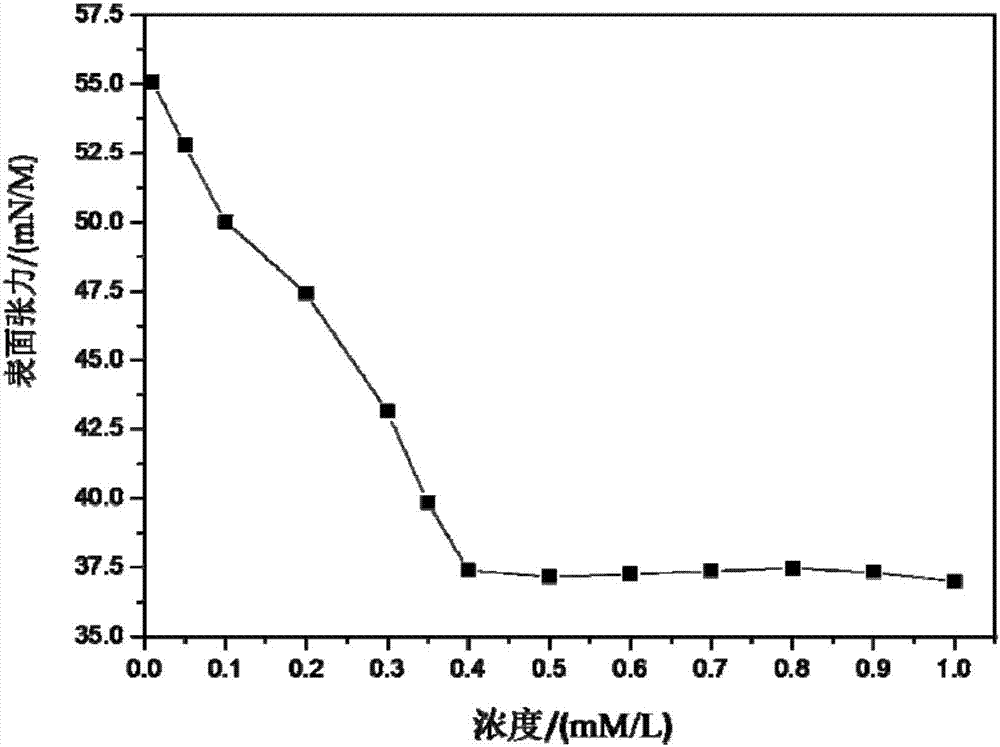
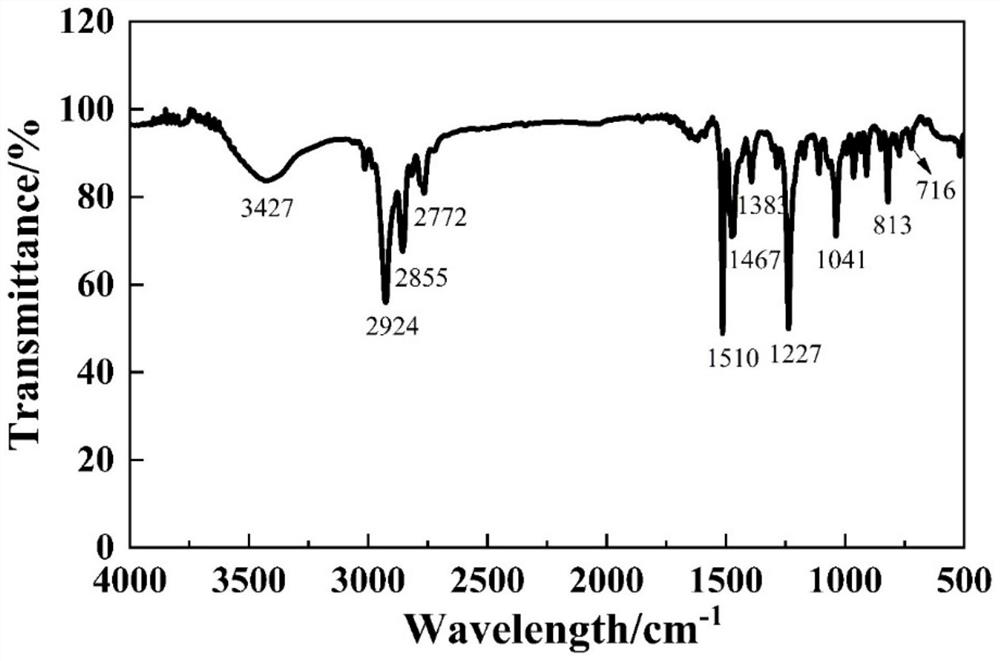
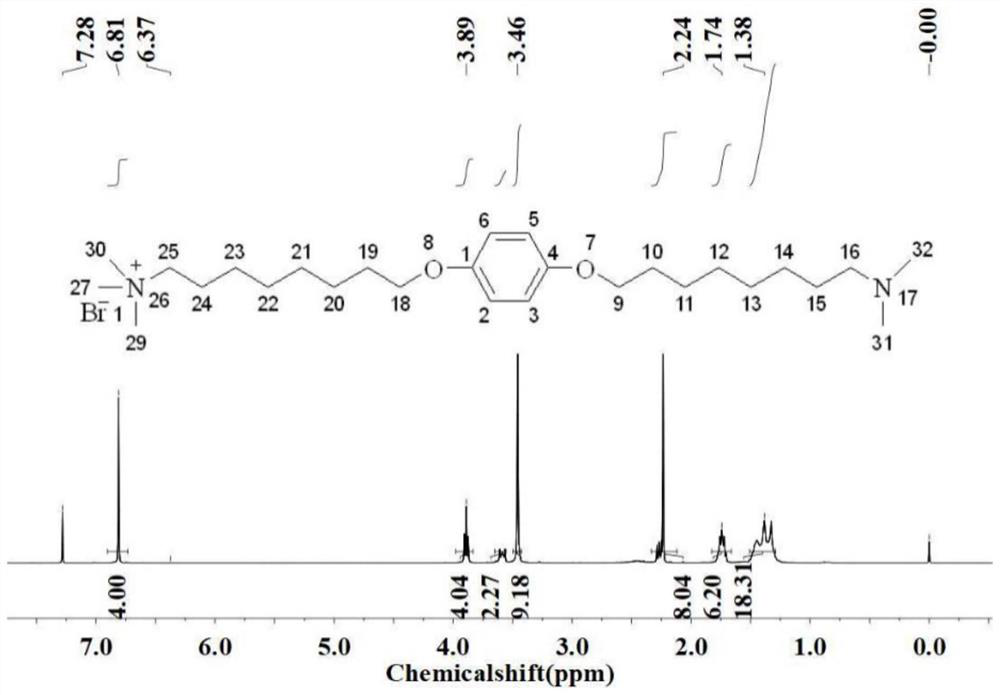
The invention discloses a photo-responsive quaternary cationic surfactant capable of forming a wormlike micelle and a synthetic method thereof. The surfactant has a structural formula as shown in the specification. In the formula, R represents a straight-chain alkyl group. The photo-responsive quaternary cationic surfactant is prepared from p-hydroxyazobenzaldehyde, 1,2-dibromoethane and N,N-dimethylalkylamine through a reduction reaction, a Williamson ether synthesis reaction and a quaterization reaction. The synthetic method provided by the invention is simple to operate and uses cheap and easily available raw materials; the synthesized surfactant has high purity and a yield of more than 64.0%; an aqueous solution of the synthesized surfactant has good photo-responsiveness and low surface tension; critical micelle concentration is lower than 4.0*10<-4> mol / L; and the synthesized surfactant can form the wormlike micelle.
Owner:SHAANXI NORMAL UNIV
Cationic surfactant containing rigid group amphipathicity-strong polarity switching
InactiveCN113480440ARealize intelligent conversionAchieve recyclingOrganic compound preparationTransportation and packagingActive agentALUMINUM HYDRIDE
The invention discloses a cationic surfactant containing rigid group amphipathicity-strong polarity switching, and belongs to the technical field of surfactant science and application. The surfactant N-nP-N<+> is synthesized by taking Br (CH2) nCOOH as a raw material through a series of reactions such as acylating chlorination, amidation, Williamson ether synthesis, lithium aluminum hydride reduction and bromination, and a hydrophobic chain of the surfactant can be intelligently switched between polarity (namely NH<+>-nP-<+>) and non-polarity (namely N-nP-N <+>). Therefore, the surfactant can be intelligently converted between amphipathicity and strong polarity, when the surfactant shows strong polarity, the surfactant can return to a water phase, the separation is more thorough due to introduction of rigid groups in a hydrophobic chain, the recovery and reutilization of the surfactant are realized, and the product can be widely applied to the fields of oil product emulsification transportation, nano material synthesis, heterogeneous catalysis and the like.
Owner:JIANGNAN UNIV
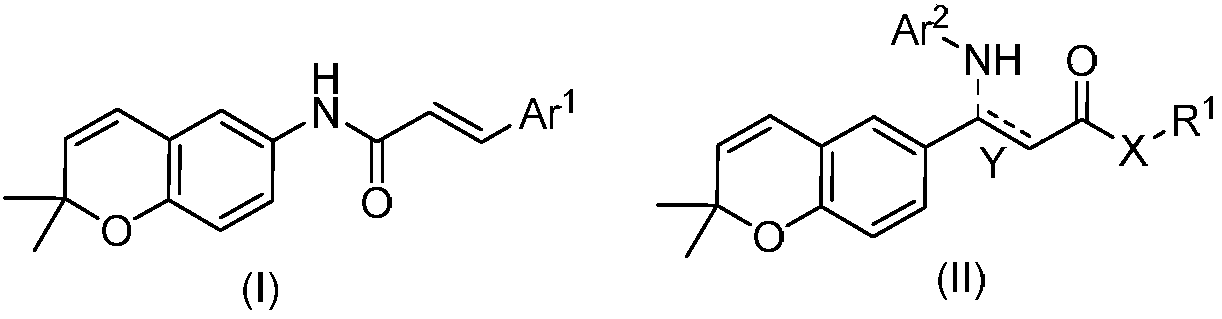
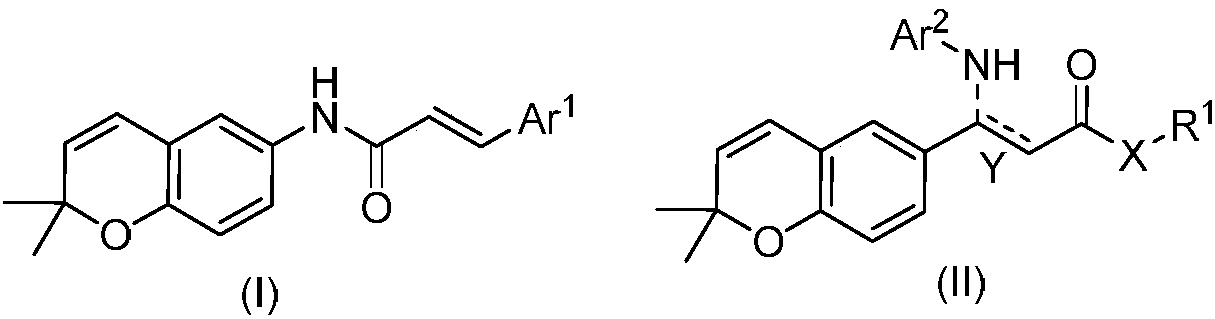
2,2-dimethylbenzopyran derivative and preparation method and use thereof
The invention belongs to the technical field of medicines, and discloses a 2,2-dimethylbenzopyran derivative and a preparation method and use of the 2,2-dimethylbenzopyran derivative. The derivative and pharmaceutically acceptable salt of the derivative have the structural formulae shown in the description, wherein Ar<1>, Ar<2>, X, Y and R1 are as described in the claims and the description. The preparation methods of the derivative and the pharmaceutically acceptable salt of the derivative comprise the following steps: the formula (I) is obtained by mainly taking acetaminophen as a raw material, and subjected to the reactions of Williamson ether formation, cyclization, hydrolysis, amide formation and the like; the formula II is obtained by mainly taking p-hydroxybenzaldehyde as a raw material, and subjected to the reactions of the Williamson ether formation, cyclization, Knoevenagel condensation, amide formation and the like. The derivative and the pharmaceutically acceptable salt ofthe derivative disclosed by the invention can be used for preparing neuroprotective agents and have better neuroprotective effects on ischemic stroke.
Owner:SHENYANG PHARMA UNIVERSITY
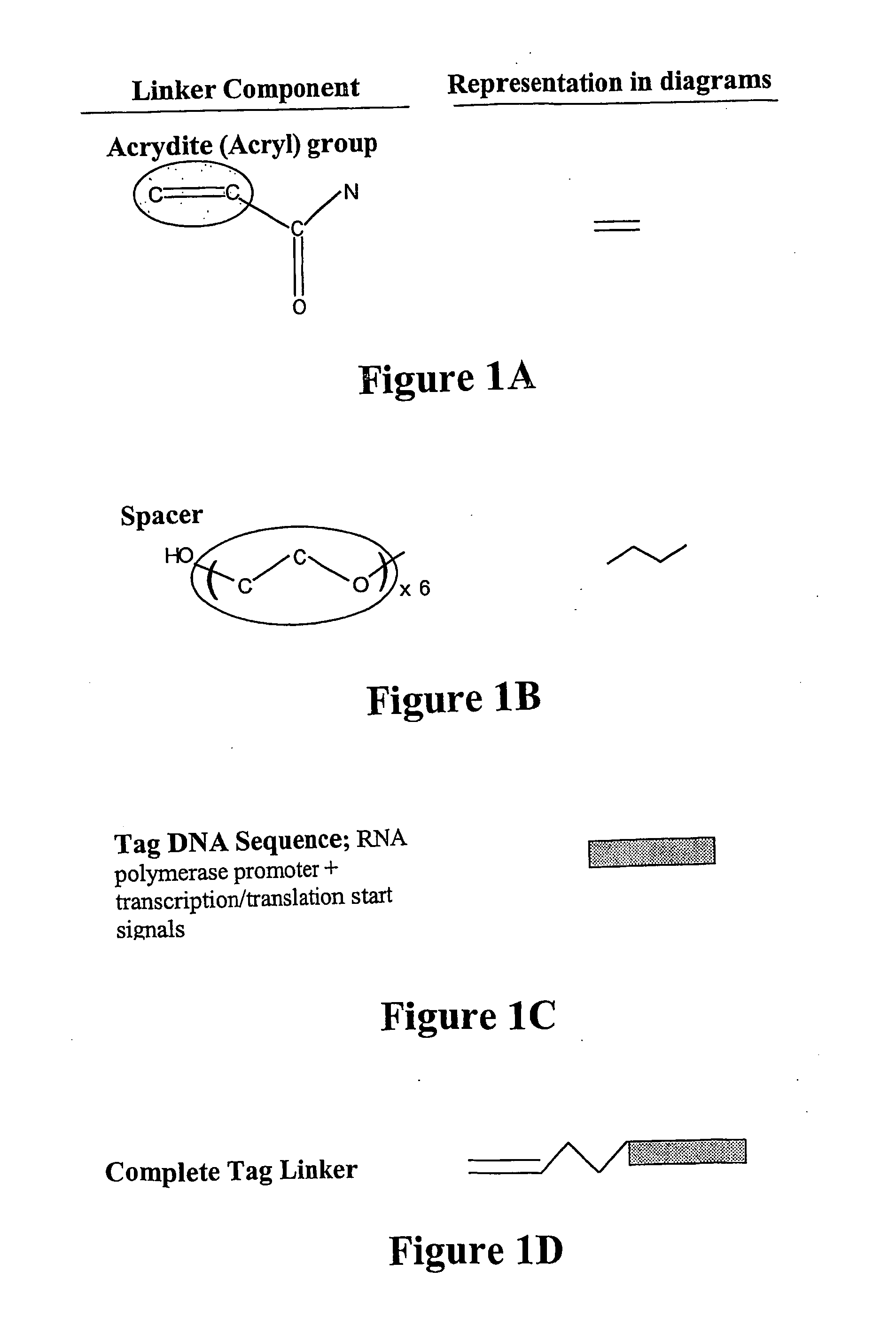
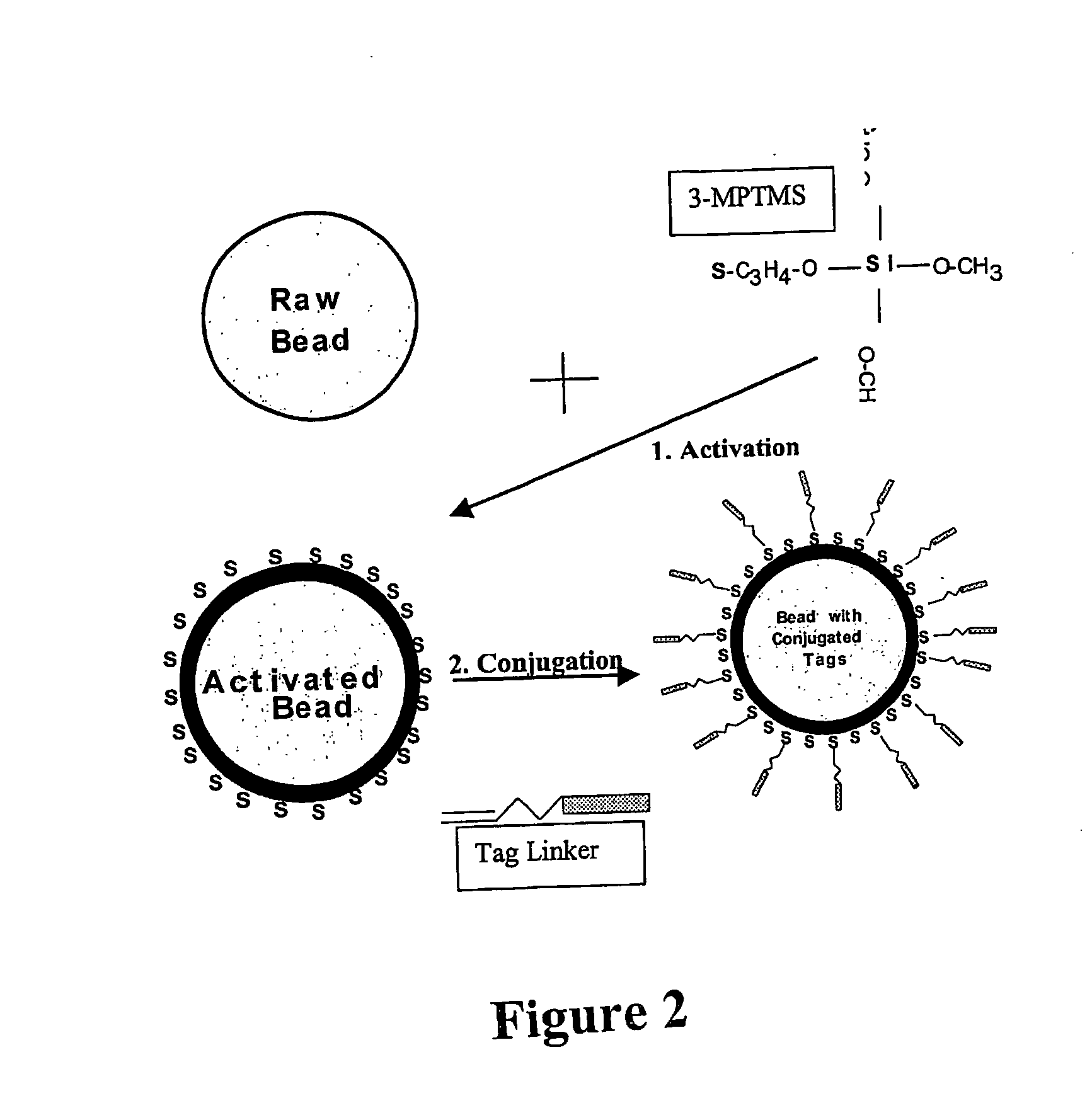
Nucleic acid anchoring system comprising covalent linkage of an oligonucleotide to a solid support
ActiveUS20060063925A1Easy to fixEasy to operateSugar derivativesMicrobiological testing/measurementChemical MoietyMicrosphere
The anchoring system generally comprises a solid support and a chemical linking moiety useful for ether formation with another chemical moiety on a nucleic acid molecule. The present invention further contemplates methods for anchoring a nucleic acid molecule to a solid support via a covalent linkage. The anchoring system of the present invention is useful inter alia in construction of nucleic acid arrays, to purify nucleic acid molecules and to anchor nucleic acid molecules so that they can be used as templates for in vitro transcription and / or translation experiments and to participate in amplification reactions. The present invention is particularly adaptable for use with microspheres and the preparation of microsphere suspension arrays and optical fiber arrays. The anchoring system permits the generation of an anchored oligonucleotide for use as a universal nucleic acid conjugation substrate for any nucleic acid molecule or population of nucleic acid molecules. The present invention further provides a kit useful for anchoring nucleic acid molecules or comprising nucleic acid molecules already anchored to a solid support.
Owner:INCITE HEALTH PTY LTD
3, 3-diazidomethyl oxetane-tetrahydrofuran energetic copolyether with alternating multi-block structure and synthesis method thereof
ActiveCN113621134AChange the microsequence structureMechanical properties can be adjustedExplosivesPolymer scienceMeth-
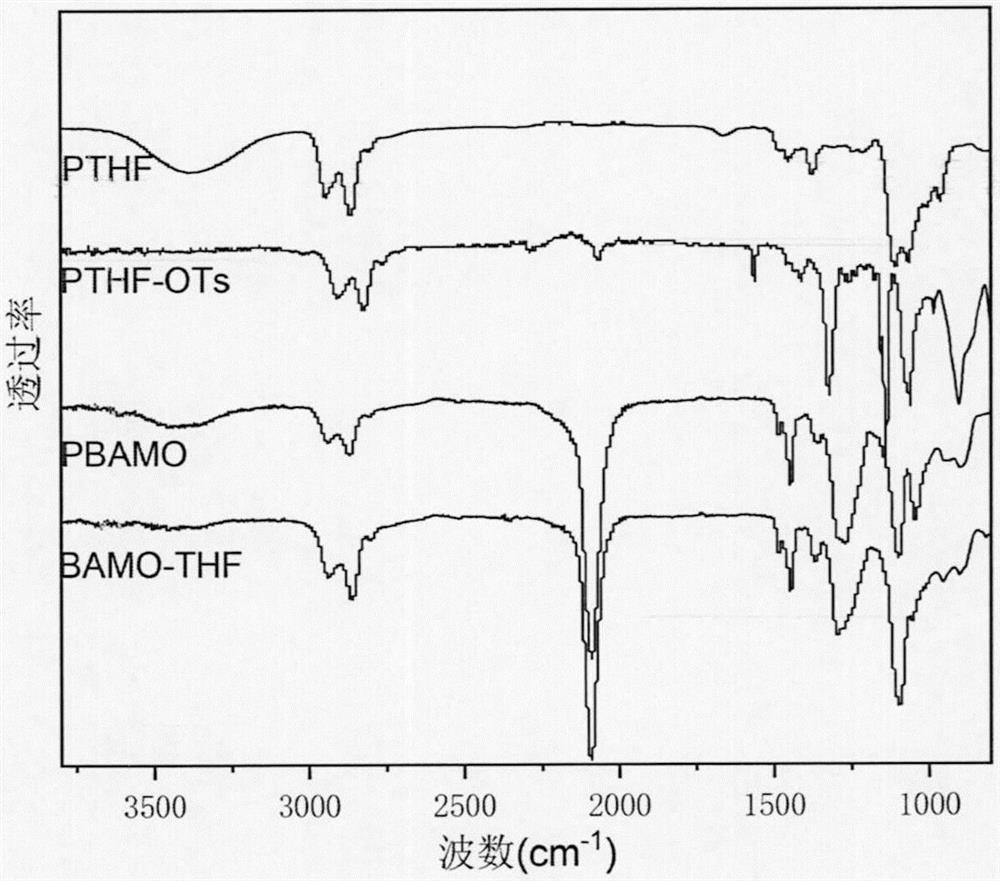
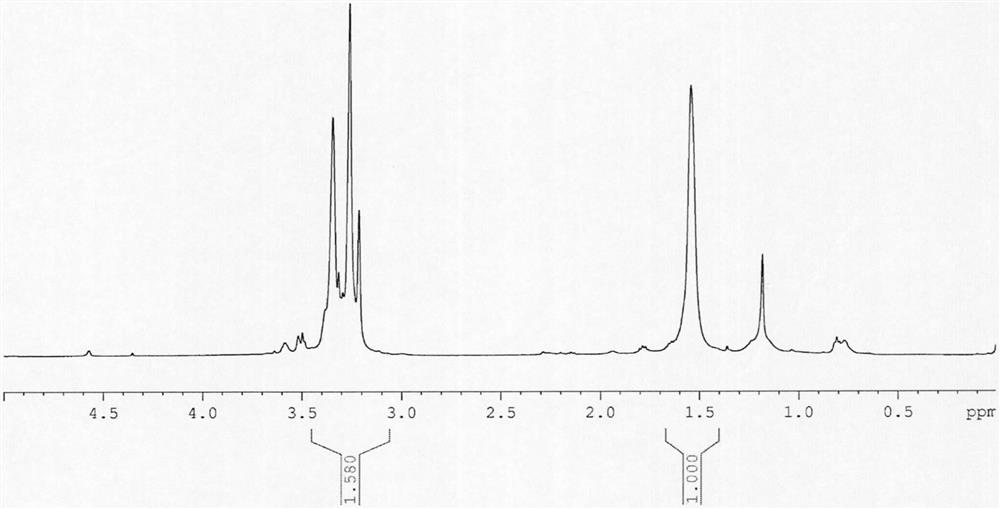
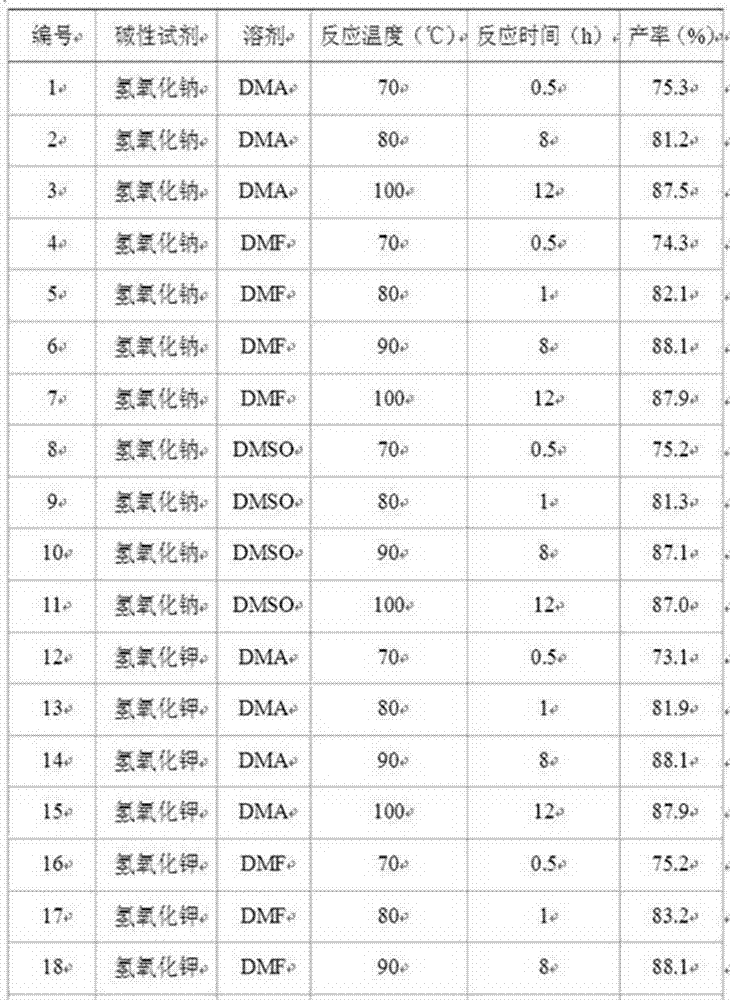
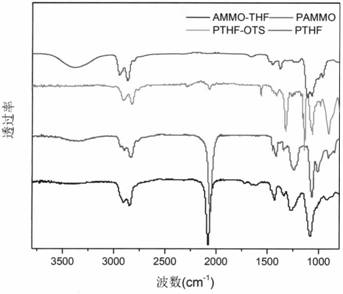
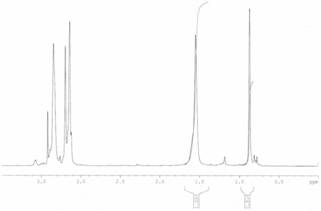
The invention discloses 3, 3-diazidomethyl oxetane-tetrahydrofuran energetic copolyether with an alternating multi-block structure and a synthesis method thereof, wherein the structural formula of the alternating multi-block energetic adhesive is shown as (I), m is equal to 1-5, n is equal to 1-4, k is equal to 1-10, and m, n and k are integers. The synthesis process comprises the following steps: by taking polytetrahydrofuran (PTHF) and a 3, 3-diazidomethyl oxetane homopolymer (PBAMO) as raw materials, carrying out Williamson ether synthesis to obtain the alternating multi-block azido energetic adhesive. The synthesis method is simple, and the alternating multi-block BAMO-THF energetic adhesive has an adjustable microscopic sequence structure and can endow a propellant with relatively good mechanical properties.
Owner:NANJING UNIV OF SCI & TECH
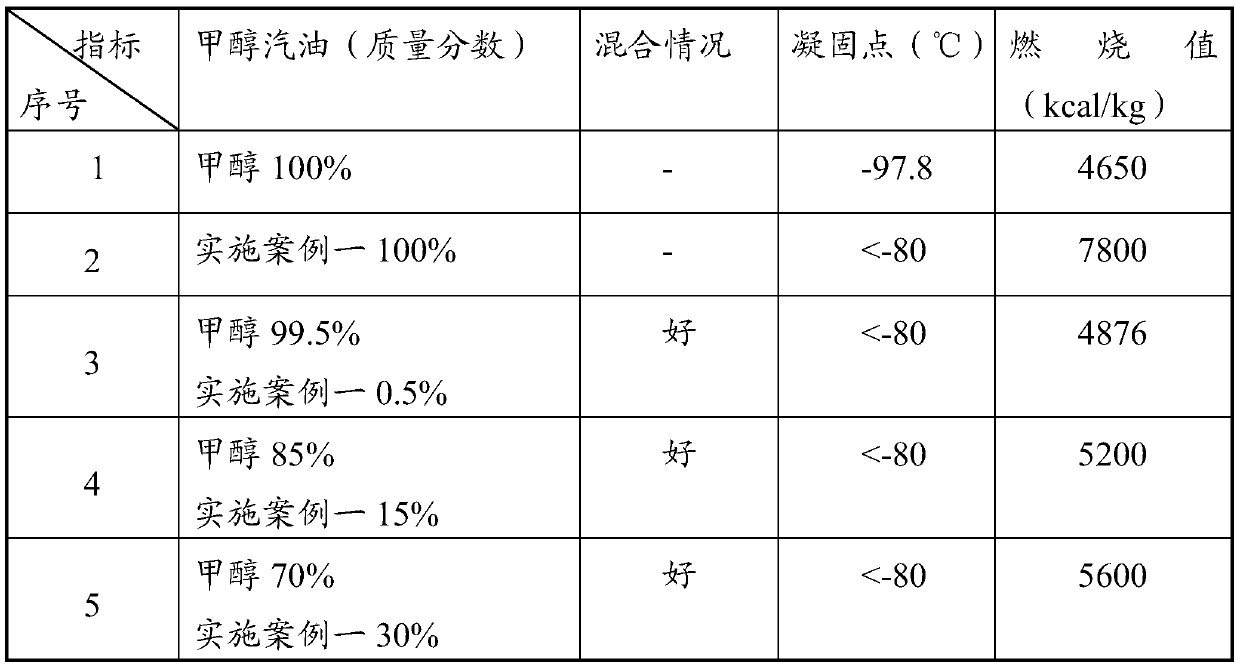
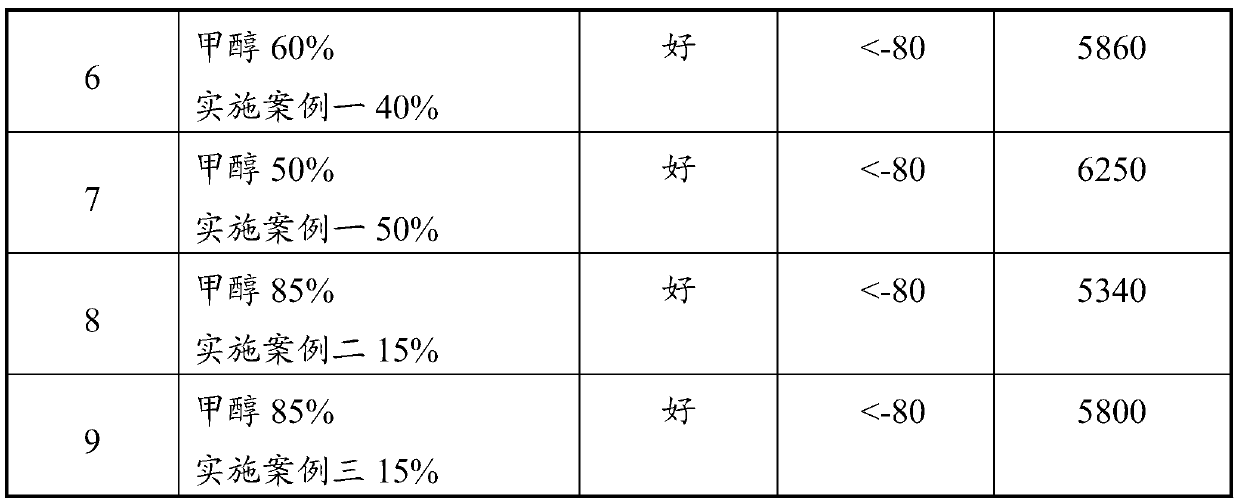
Methanol gasoline additive, preparation method thereof and methanol gasoline containing additive
InactiveCN110240950AHigh combustion valueThere will be no stratificationLiquid carbonaceous fuelsCombustionGlycol ethers
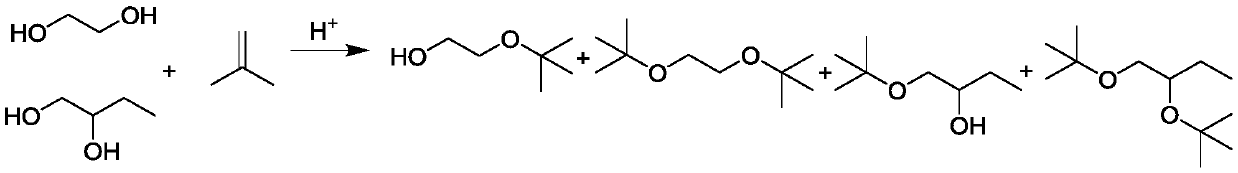
The invention discloses a methanol gasoline additive and a preparation method thereof, and relates to the technical field of gasoline synthesis. The additive is a glycol ether mixture and can be dissolved with methanol in any ratio. The freezing point is -80 DEG C or lower, so that layering cannot occur even in a low-temperature environment. The glycol ether has a higher combustion value than methanol, and can greatly improve the combustion value of methanol gasoline when being added into the methanol gasoline as a methanol additive. Raw materials in the preparation method are selected to be ethylene glycol and / or 1,2-butanediol for carrying out an ether formation reaction with a tert-butyl donor. The process is simple, production cost is low, and industrial production values are achieved. The methanol gasoline containing the additive has the characteristics that stability is good, the combustion value is high, and the methanol gasoline can be used under an ultralow-temperature condition.
Owner:深圳市前海博扬研究院有限公司
Preparation method for 4-aminoacetophenone
InactiveCN102924306AMild reaction conditionsEasy to operateOrganic chemistryOrganic compound preparationM-aminoacetophenoneEngineering
The invention belongs to the medical technical field, and relates to a bran-new synthetic route for preparing 4-aminoacetophenone. Through a Williamson ether synthesis method and Smiles rearrangement reaction, benzamide compounds are produced, then the 4-aminoacetophenone preparation is finished after hydrolysis. Reaction condition is gentle, and flammable and combustible reagents and high-toxic reagents are not involved. Yield coefficient is higher than that of the preparation method in documents. The preparation method is an economical and practical synthetic method, and suitable for large-scale industrial production.
Owner:SHENYANG PHARMA UNIVERSITY
3-azido methyl-3-methyloxetane-tetrahydrofuran energetic copolyether with alternating multi-block structure and synthesis method thereof
ActiveCN113501963APolycondensationChange the microsequence structureExplosivesPressure gas generationPolymer sciencePtru catalyst
The invention discloses 3-azidomethyl-3-methyloxetane-tetrahydrofuran energetic copolyether with an alternating multi-block structure and a synthesis method thereof. The structural formula of the alternating multi-block energetic adhesive is shown as (I) in the specification, wherein m is equal to 1-5, n is equal to 1-4, k is equal to 1-10, and m, n and k are integers. The synthesis process comprises the following step of: by taking polytetrahydrofuran (PTHF) and a 3-azidomethyl-3-methyloxetane homopolymer (PAMMO) as raw materials and KOH as a catalyst, carrying out a Williamson ether synthesis method to obtain the alternate multi-block azido energetic adhesive. The synthesis method is simple, and the alternating multi-block AMMO-THF energetic adhesive has an adjustable microscopic sequence structure and can endow a material with relatively good mechanical properties and processability.
Owner:NANJING UNIV OF SCI & TECH
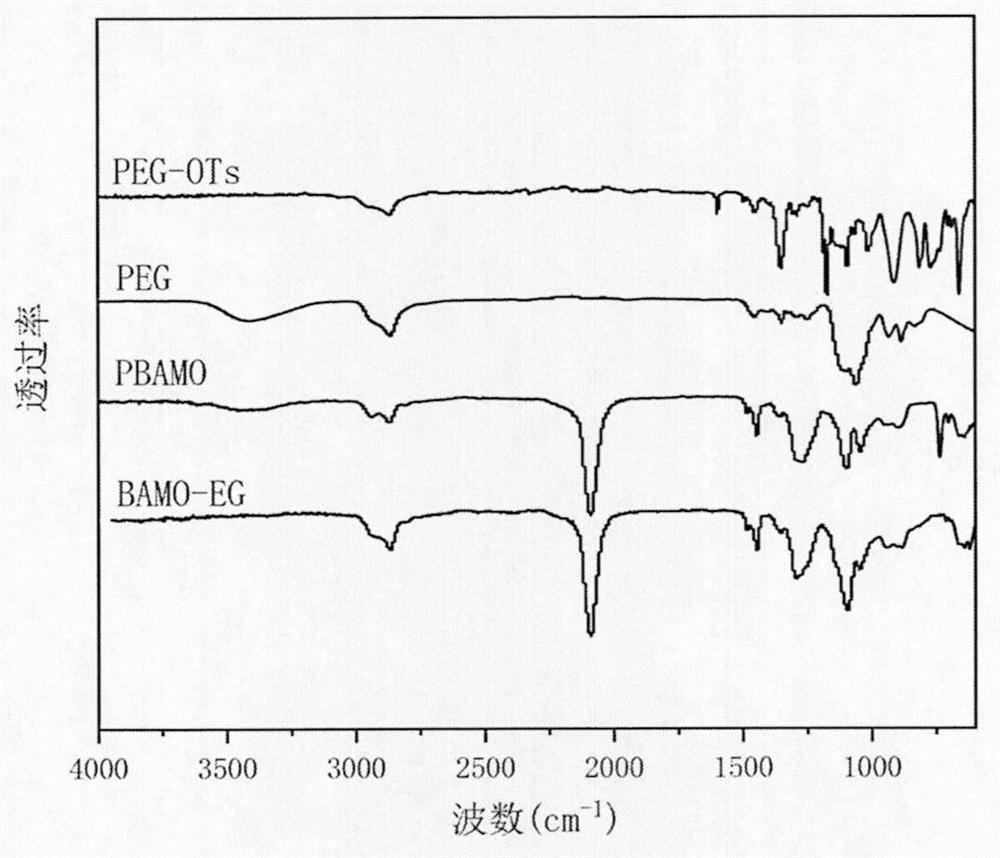
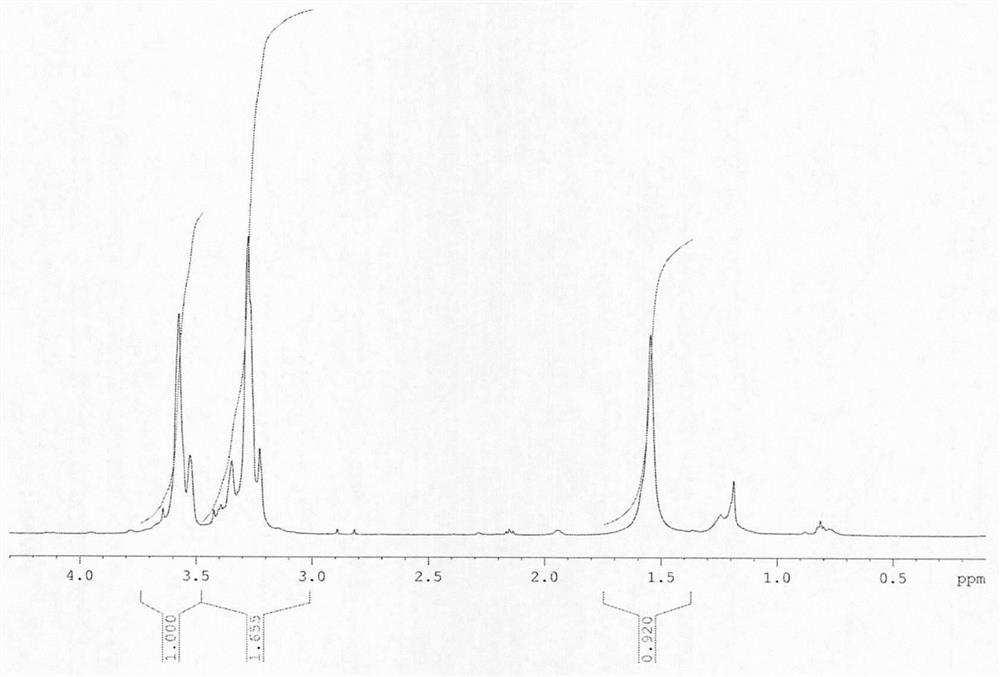
3, 3-diazido methyl oxetane-ethylene glycol energetic copolyether with alternating multi-block structure and synthesis method thereof
PendingCN114133553APolycondensationNon-explosive/non-thermic compositionsPressure gas generationPolymer scienceAdhesive
The invention discloses 3, 3-diazido methyl oxetane-ethylene glycol energetic copolyether with an alternating multi-block structure and a synthesis method thereof, the structural formula of an alternating multi-block energetic adhesive is shown as (I), m is equal to 1-4, n is equal to 1-4, l is equal to 1-4, k is equal to 1-10 and is an integer. The synthesis process comprises the following steps: by taking polyethylene glycol (PEG) and a 3, 3-diazido methyl oxetane homopolymer (PBAMO) as raw materials, carrying out Williamson ether synthesis to obtain the alternate multi-block azido energetic copolyether. The synthesis method is simple, and the alternating multi-block BAMO-EG energetic copolyether has an adjustable microscopic sequence structure and can endow a propellant with relatively good mechanical properties.
Owner:NANJING UNIV OF SCI & TECH
Preparation method of multi-target anti-tumor drug
ActiveCN112159351AHigh reaction yieldHigh yieldOrganic chemistryAntineoplastic agentsAcyl groupPhenyl group
The invention relates to a preparation method of a multi-target anti-tumor drug. The method comprises the following steps: 1, nitrogen protection: by using a williamson ether synthesis method, using 4-amino-3-fluorophenol and 4-chlorine-2-pyridine carboxamide as raw materials and dimethyl sulfoxide and tetrahydrofuran as a mixed solvent under the effect of potassium tert-butoxide to generate 2-carbamoyl-4-((3-fluorine-4-amino) phenoxy) pyridine by reaction; and 2, reacting the 2-carbamoyl-4-((3-fluorine-4-amino) phenoxy) pyridine with 4-chlorine-3-(trifluoromethyl) benzene isocyanate in a dioxane solvent to obtain a crude product, and refining the crude product to obtain a final product 4-{4-[3-(4-chlorine-3-trifluoromethyl-phenyl)urea]-3-fluorine phenoxy} pyridine-2-formamide.
Owner:GUANGZHOU NUCIEN PHARM CO LTD +1
Multi-step synthesis method for 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepine
ActiveCN108997250ARaw materials are easy to getMild reaction conditionsOrganic chemistryOrganic baseOrganic synthesis
The invention discloses a multi-step synthesis method for 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepine, and belongs to the technical field of organic synthesis. The method comprises the following steps:2-nitrobenzyl alcohol and propargyl bromide or propargyl alcohol are used for ether formation, and 1-nitro-2-(propynyloxymethyl)-benzene is obtained; then, reduction is performed with iron powder / acetic acid or NiCl2 (dppp) / tetrahydroxydiboron / organic base, and 2-(propynyloxymethyl)-aniline is obtained; 2-(propynyloxymethyl)-aniline is subjected to Sonogashira coupling with iodobenzene, and 2-[(3-phenyl-2-alkynyloxy)methyl]-aniline is obtained; amino is subjected to p-toluenesulfonyl protection, cuprous bromide / cesium carbonate ring closing is performed, deprotection is performed under the condition of metal sodium / naphthalene, and 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepine is obtained.
Owner:山东三牧新材料科技有限公司
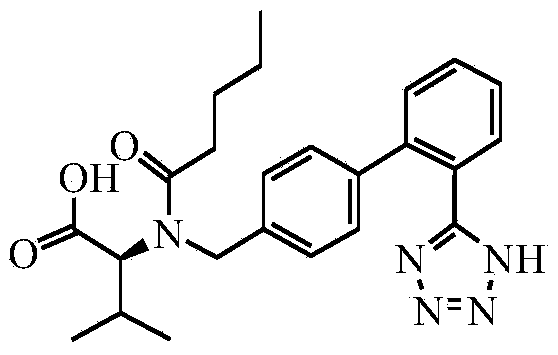
Method for preparing valsartan
InactiveCN104292174AAvoid racemizationGuaranteed optical purityOrganic chemistrySodium methoxideValsartan
The invention provides a method for preparing valsartan. The method comprises the following steps: firstly, reacting N-(triphenylmethyl)-5-(4'-formyl-biphenyl-2-yl) tetrazolium with sodium methoxide, secondly, adding dichlorodicyanobenzoquinone (DDQ) and oxidizing, thirdly, reacting with L-valine methyl ester, fourthly, alkylating with valeryl chloride, fifthly, removing triphenylmethyl group and sixthly removing ester group and acidifying to obtain the target product valsartan. According to the method, a novel method for preparing valsartan is provided by virtue of the Williamson ether synthesis step and DDQ oxidation step, since potassium dihydrogen phosphate is adopted as a buffering reagent in the triphenylmethyl group removal process and a 1.3mol / L sodium hydroxide solution is used in the hydrolysis process, the problem of racemization of the product generated caused by too strong alkaline is avoided and the method is of important significance in maintaining the optical purity of the valsartan product.
Owner:EAST CHINA UNIV OF SCI & TECH
Nucleic acid anchoring system comprising covalent linkage of an oligonucleotide to a solid support
ActiveUS7741459B2Easy to fixEasy to operateSugar derivativesMicrobiological testing/measurementChemical MoietyChemical ligation
The anchoring system generally comprises a solid support and a chemical linking moiety useful for ether formation with another chemical moiety on a nucleic acid molecule. The present invention further contemplates methods for anchoring a nucleic acid molecule to a solid support via a covalent linkage. The anchoring system of the present invention is useful inter alia in construction of nucleic acid arrays, to purify nucleic acid molecules and to anchor nucleic acid molecules so that they can be used as templates for in vitro transcription and / or translation experiments and to participate in amplification reactions. The present invention is particularly adaptable for use with microspheres and the preparation of microsphere suspension arrays and optical fiber arrays. The anchoring system permits the generation of an anchored oligonucleotide for use as a universal nucleic acid conjugation substrate for any nucleic acid molecule or population of nucleic acid molecules. The present invention further provides a kit useful for anchoring nucleic acid molecules or comprising nucleic acid molecules already anchored to a solid support.
Owner:INCITE HEALTH PTY LTD
Synthesis method of abiraterone
ActiveCN103360458BLow priceEasy to getSteroidsBulk chemical productionOrganic solventGrignard reagent
Owner:BRIGHTGENE PHARMA
Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine
ActiveCN108997251BRaw materials are easy to getMild reaction conditionsOrganic chemistryMethylanilineOrganic synthesis
Owner:上海三牧化工技术有限公司
Method for multi-step synthesis of 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepines
ActiveCN108997250BRaw materials are easy to getMild reaction conditionsOrganic chemistryMethylanilineOrganic synthesis
The invention discloses a multi-step synthesis method for 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepine, and belongs to the technical field of organic synthesis. The method comprises the following steps:2-nitrobenzyl alcohol and propargyl bromide or propargyl alcohol are used for ether formation, and 1-nitro-2-(propynyloxymethyl)-benzene is obtained; then, reduction is performed with iron powder / acetic acid or NiCl2 (dppp) / tetrahydroxydiboron / organic base, and 2-(propynyloxymethyl)-aniline is obtained; 2-(propynyloxymethyl)-aniline is subjected to Sonogashira coupling with iodobenzene, and 2-[(3-phenyl-2-alkynyloxy)methyl]-aniline is obtained; amino is subjected to p-toluenesulfonyl protection, cuprous bromide / cesium carbonate ring closing is performed, deprotection is performed under the condition of metal sodium / naphthalene, and 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepine is obtained.
Owner:山东三牧新材料科技有限公司
Hydrogenation catalysts prepared from polyoxometalate precursors and process for using same to produce ethanol while minimizing diethyl ether formation
InactiveUS8658843B2Organic compound preparationHeterogenous catalyst chemical elementsAlcoholDiethyl ether
The present invention relates to hydrogenation catalysts prepared from polyoxometalate precursors. The polyoxometalate precursors introduce a support modifier to the catalyst. The catalysts are used for hydrogenating alkanoic acids and / or esters thereof to alcohols with relatively low ether formation, preferably with conversion of the ester coproduct. The catalyst may also comprise one or more active metals.
Owner:CELANESE INT CORP
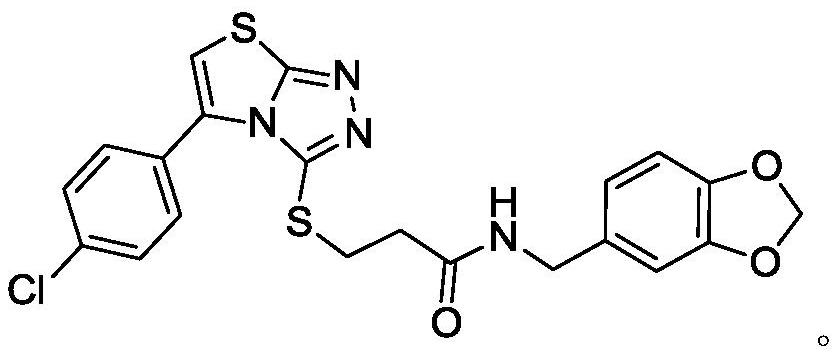
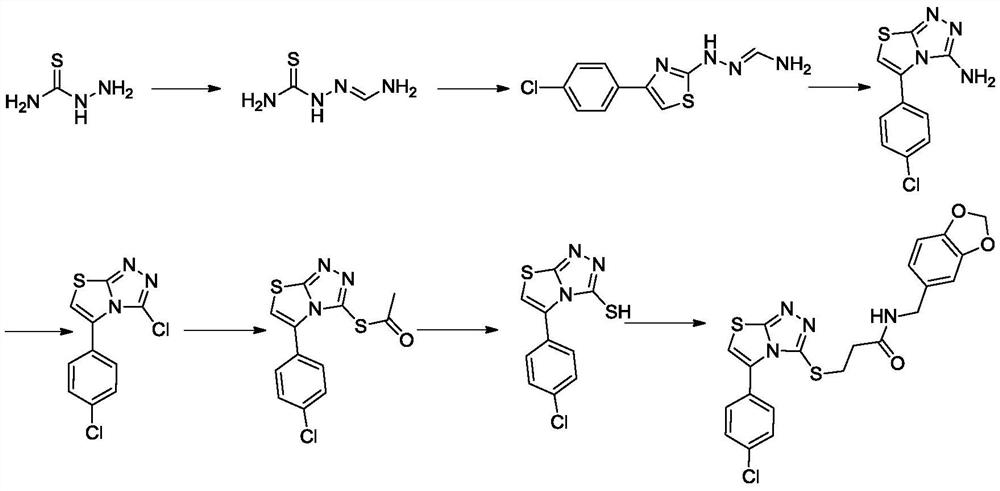
A kind of thiazolotriazole superoxide dismutase agonist and its preparation method and application
The invention discloses a thiazolotriazole superoxide dismutase agonist, a preparation method and application thereof, and belongs to the technical field of synthesis of protease agonists. The gist of the technical solution of the present invention is as follows: the thiazolotriazole superoxide dismutase agonist has the structure of Intramolecular ring formation, chlorination, ester formation, ester hydrolysis, ether formation and other multi-step reactions obtain novel thiazolotriazole molecules. The invention is simple and quick to operate, has high yield, is suitable for large-scale industrial production, and the thiazolotriazole molecules can effectively improve the activity of SOD in cosmetics, and have the potential to become auxiliary additives for cosmetics.
Owner:九吉兴(北京)生物科技有限公司
A kind of synthetic method of glabridin
Owner:SHAANXI NORMAL UNIV
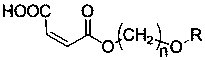
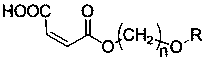
Maleate monomers used for preparing pour depressant components
InactiveCN107759470AEasy to prepareRaw materials are easy to getOrganic compound preparationCarboxylic acid esters preparationAlkaneAlcohol
The invention discloses maleate monomers used for preparing pour depressant components and a preparation method of the maleate monomers. The maleate monomers are structurally characterized by being maleate alkoxy alcohol ester. The preparation method comprises the steps that n-alcohol and n-alkane with the terminal substituted with dichloro are used as raw materials, chloroether with the total number of carbon of 20-26 is synthesized through the Williamson ether synthesis method, and then the chloroether and sodium maleate are subjected to the SN2 reaction to synthesize maleate. The preparation method of the maleate monomers is simple, the raw materials are easy to obtain, the product yield is high, and many kinds of maleate monomers can be prepared.
Owner:天津市明瑞石油科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthesis method of 2-[(-4-chlorophenyl)(4-piperidinyl-oxy)methyl]pyridine having single optical isomer Synthesis method of 2-[(-4-chlorophenyl)(4-piperidinyl-oxy)methyl]pyridine having single optical isomer](https://images-eureka.patsnap.com/patent_img/b50fa5c1-760d-4f30-a249-27959511c3bd/BDA00002897357400011.PNG)
![Synthesis method of 2-[(-4-chlorophenyl)(4-piperidinyl-oxy)methyl]pyridine having single optical isomer Synthesis method of 2-[(-4-chlorophenyl)(4-piperidinyl-oxy)methyl]pyridine having single optical isomer](https://images-eureka.patsnap.com/patent_img/b50fa5c1-760d-4f30-a249-27959511c3bd/BDA00002897357400012.PNG)
![Synthesis method of 2-[(-4-chlorophenyl)(4-piperidinyl-oxy)methyl]pyridine having single optical isomer Synthesis method of 2-[(-4-chlorophenyl)(4-piperidinyl-oxy)methyl]pyridine having single optical isomer](https://images-eureka.patsnap.com/patent_img/b50fa5c1-760d-4f30-a249-27959511c3bd/BDA00002897357400021.PNG)


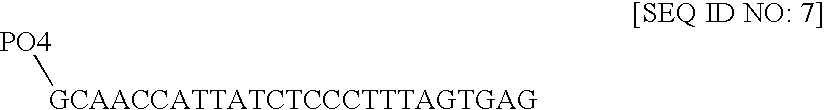

![Multi-step synthesis method for 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepine Multi-step synthesis method for 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepine](https://images-eureka.patsnap.com/patent_img/0de04abe-1ae5-4a1c-9662-b49d58c49723/BDA0001705503450000021.png)
![Multi-step synthesis method for 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepine Multi-step synthesis method for 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepine](https://images-eureka.patsnap.com/patent_img/0de04abe-1ae5-4a1c-9662-b49d58c49723/BDA0001705503450000061.png)
![Multi-step synthesis method for 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepine Multi-step synthesis method for 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepine](https://images-eureka.patsnap.com/patent_img/0de04abe-1ae5-4a1c-9662-b49d58c49723/BDA0001705503450000091.png)
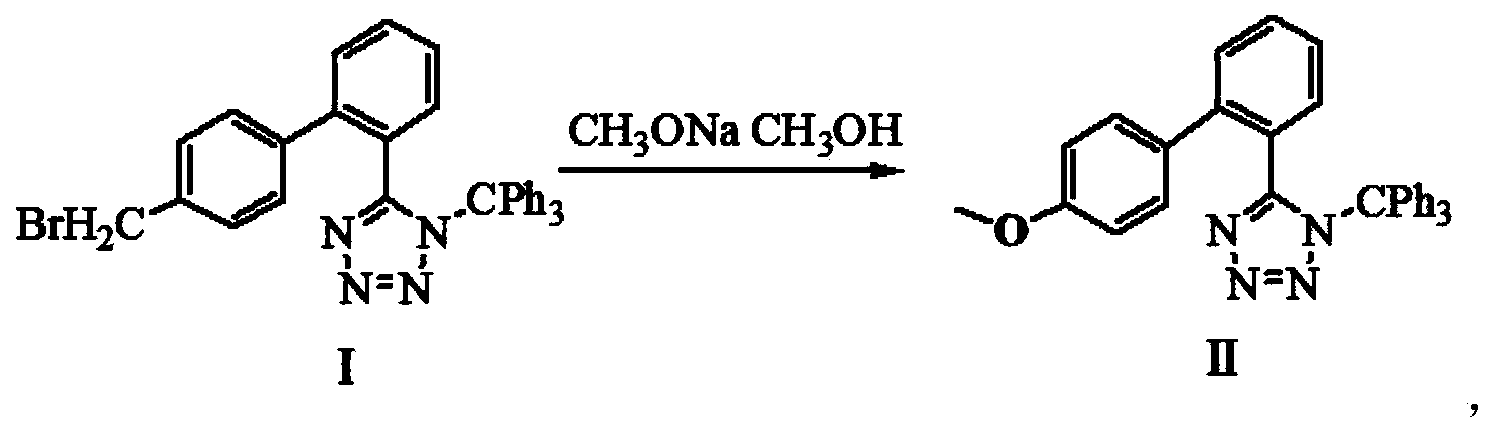
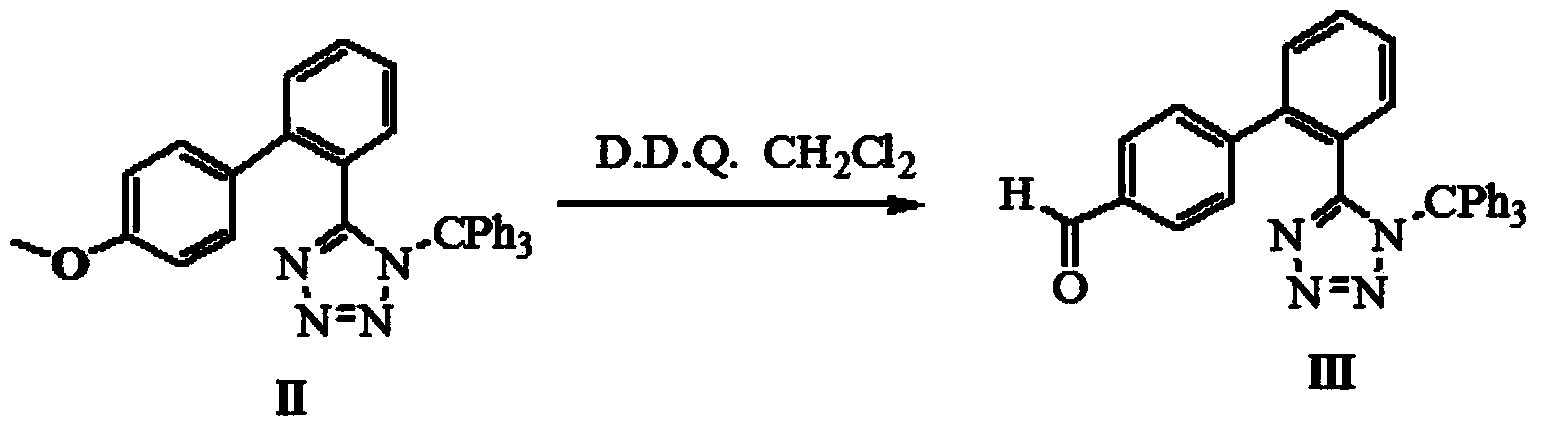
![Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine](https://images-eureka.patsnap.com/patent_img/441aef19-333a-4fee-bcf5-3684c2e3934b/BDA0001705504470000021.png)
![Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine](https://images-eureka.patsnap.com/patent_img/441aef19-333a-4fee-bcf5-3684c2e3934b/BDA0001705504470000061.png)
![Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine Method for synthesizing (e)-2-benzylidene-1,2,3,5-tetrahydrobenzo[e][1,4]oxazepine](https://images-eureka.patsnap.com/patent_img/441aef19-333a-4fee-bcf5-3684c2e3934b/BDA0001705504470000091.png)
![Method for multi-step synthesis of 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepines Method for multi-step synthesis of 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepines](https://images-eureka.patsnap.com/patent_img/4df1b57e-ffcb-4764-8d7f-22a916ac83bd/BDA0001705503450000021.png)
![Method for multi-step synthesis of 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepines Method for multi-step synthesis of 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepines](https://images-eureka.patsnap.com/patent_img/4df1b57e-ffcb-4764-8d7f-22a916ac83bd/BDA0001705503450000061.png)
![Method for multi-step synthesis of 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepines Method for multi-step synthesis of 2-benzyl-1,5-dihydrobenzo[e][1,4]oxazepines](https://images-eureka.patsnap.com/patent_img/4df1b57e-ffcb-4764-8d7f-22a916ac83bd/BDA0001705503450000091.png)