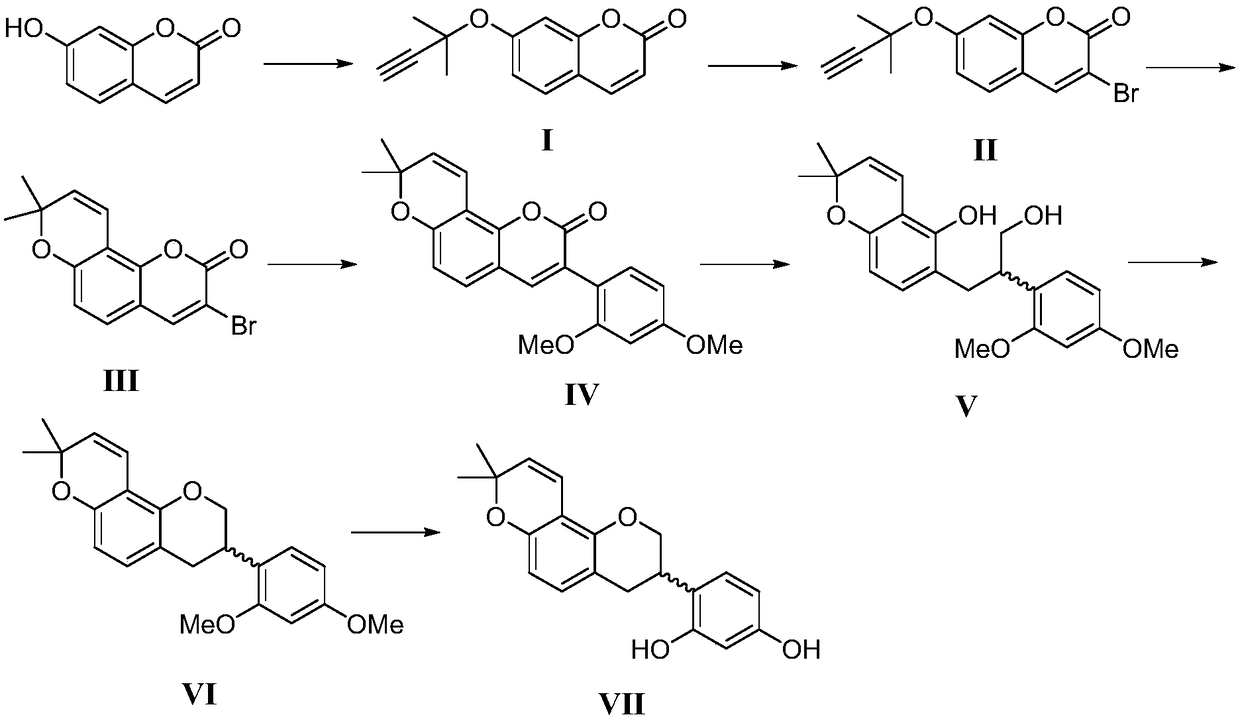
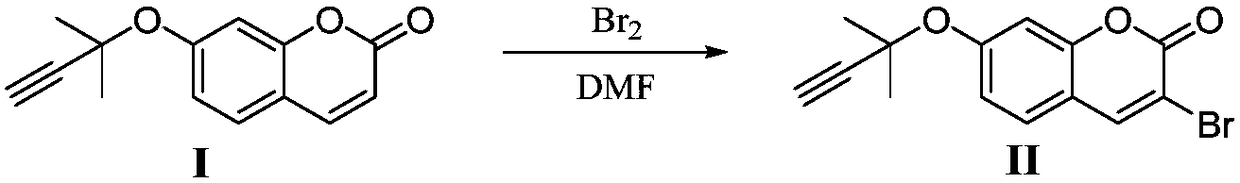
Synthesis method of glabridin
A technology of glabridin and a synthetic method, applied in directions such as organic chemistry, can solve problems such as long cycle, and achieve the effects of high total yield, fast and efficient synthesis, and short total route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
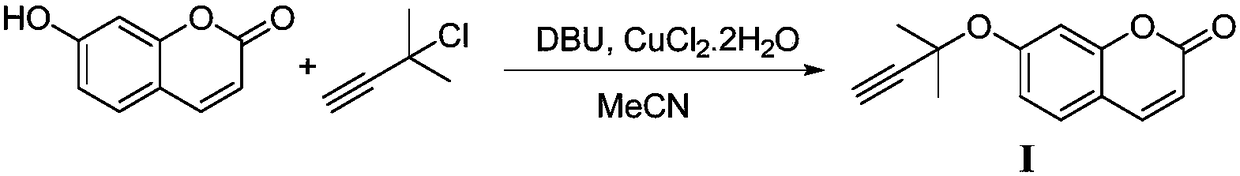
[0025] 1. Add 0.30g (1.85mmol) of 7-hydroxycoumarin into a 10mL round bottom flask, add 3mL of acetonitrile, 0.31mL (2.04mmol) of DBU and 0.32mg (0.00185mmol) of copper chloride dihydrate in turn, at 0°C Slowly add 0.22mL (1.94mmol) of 2-methyl-3-butyn-1-ol, react at room temperature for 10h, and TLC monitors that the reaction of raw materials is complete. Pour the reaction solution into saturated NaHCO 3 In aqueous solution, after extraction with ethyl acetate, over anhydrous MgSO 4 Dry, filter, concentrate under reduced pressure, and separate by column chromatography (eluent: EA / PE=1:3) to obtain compound I (0.37g, yield 90%, white solid, Mp: 253-255°C), reaction equation As follows:
[0026]
[0027] The structural characterization data of the obtained compound I are: 1 H NMR (400MHz, CDCl 3 )δ7.64(d, J=9.2Hz, 1H), 7.36(d, J=8.8Hz, 1H), 7.32(d, J=2.0Hz, 1H), 7.04(dd, J=8.4, 2.0Hz, 1H), 6.28(d, J=9.6Hz, 1H), 2.66(s, 1H), 1.71(s, 6H); HRMS(ESI) m / z: theoretical value ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com