Synthesis method of abiraterone
A technology of abiraterone and synthetic method, which is applied in the production of steroids, bulk chemicals, organic chemistry, etc., can solve the problems of many by-products, high risk and toxicity, high price, etc., and achieve easy operation, quantity and The effect of content control and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
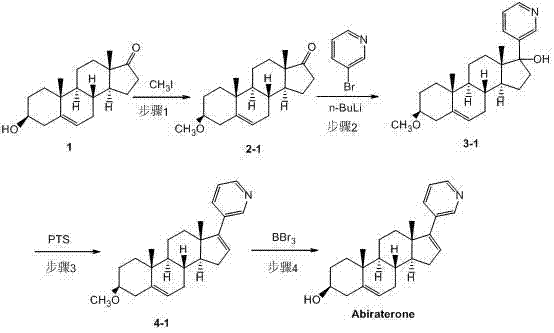
[0040] step 1:
[0041] Dissolve 2.32g of compound 1 (dehydroepiandrosterone) in 50ml of anhydrous THF, add 0.22g of sodium hydride at room temperature, stir for 30 minutes, slowly inject 2.5ml of iodomethane (2.28g / ml), and then heat to Stir at 40°C for 2.5 hours, cool the reaction solution to room temperature, add 150ml of water to the reaction solution, extract with ethyl acetate (3×50ml), combine the organic layers, dry over anhydrous magnesium sulfate, filter the magnesium sulfate, evaporate solvent to obtain 2.13 g of crude compound 2-1 ((3β)-methoxydehydroepiandrosterone), with a yield of 88.4%.
[0042] 1 H-NMR (400 MHz, CDCl 3 ) δ =0.88 (s, 3H), 0.96-1.05 (m, 1H), 1.03 (s, 3H), 1.04-1.14 (m, 1H), 1.28-1.32 (m, 2H), 1.45-1.56 (m, 4H) , 1.62-1.71 (m, 3H), 1.81-1.90 (m, 3H), 1.91-1.98 (m, 1H), 2.06-2.14 (m, 2H), 2.21-2.36 (m, 2H), 2.43-2.49 ( dd, J =15.2, 8.0 Hz, 1H), 3.15 (s, 3H), 3.18-3.19 (m, 1H), 5.39 (d, J =2.0Hz, 1H)
[0043] Step 2:
[0044] ...
Embodiment 2
[0053]
[0054] step 1:
[0055] Dissolve 2.35g of compound 1 in 30ml of anhydrous THF, add 0.24g of sodium hydride at room temperature, stir for 30 minutes, dissolve 1.12g of MEMCl in 15ml of anhydrous THF, and slowly add it dropwise to the reaction solution. Above 0°C, stir for 1 hour, after rising to room temperature, add 150ml of water to the reaction solution, extract with ethyl acetate (3×50ml), combine the organic layers, dry over anhydrous magnesium sulfate, filter out anhydrous magnesium sulfate, The solvent was evaporated to obtain 2.88 g of crude product of compound 2-2 ((3β)-methoxyethoxymethoxy-17-(3-pyridyl)-androst-5-en-17-ol), yield 93.9 %.
[0056] 1 H-NMR (400 MHz, CDCl 3 ) δ =0.89 (s, 3H), 0.94-1.04 (m, 1H), 1.03 (s, 3H), 1.06-1.14 (m, 1H), 1.27-1.31 (m, 2H), 1.46-1.56 (m, 4H) , 1.64-1.74 (m, 3H), 1.80-1.90 (m, 3H), 1.92-1.98 (m, 1H), 2.03-2.12 (m, 2H), 2.22-2.36 (m, 2H), 2.43-2.48 ( dd, J =15.2, 8.0 Hz, 1H), 3.20 (s, 3H), 3.25-3.26 (m, 1H), 3.28-...
Embodiment 3
[0066]
[0067] step 1:
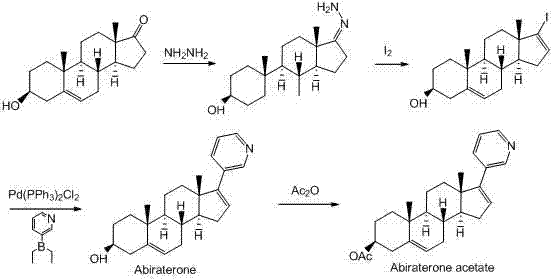
[0068] Dissolve 2.54g of compound 1 in 30ml of pyridine, then slowly drop into 1.91g of TMSCl (trimethylchlorosilane), stir at room temperature for 3 hours, add 60ml of water to the reaction solution, and then use ethyl acetate (3×50ml ) to extract the aqueous layer, combine the organic layers, wash the organic layer with 50ml of water and 50ml of saturated brine, and add anhydrous magnesium sulfate to dry overnight to obtain 2.86g of compound 2-3 ((3β)-trimethylsilyloxy de Hydrogen epiandrosterone) crude product, yield 90.0%.
[0069] 1 H-NMR (400 MHz, CDCl 3 ) δ =0.31 (s, 9H), 0.95 (s, 3H), 0.95-1.05 (m, 1H), 1.05 (s, 3H), 1.07-1.15 (m, 1H), 1.29-1.32 (m, 2H), 1.48 -1.57 (m, 4H), 1.67-1.76 (m, 3H), 1.85-1.99 (m, 4H), 2.05-2.17 (m, 2H), 2.28-2.39 (m, 2H), 2.45-2.51 (m, 1H), 3.25-3.26(m, 1H), 5.32(d, J =2.0Hz, 1H)
[0070] Step 2:
[0071] First, dissolve 0.43g of isopropylmagnesium chloride in 15ml of anhydrous THF, cool to 0°C in a low-t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com