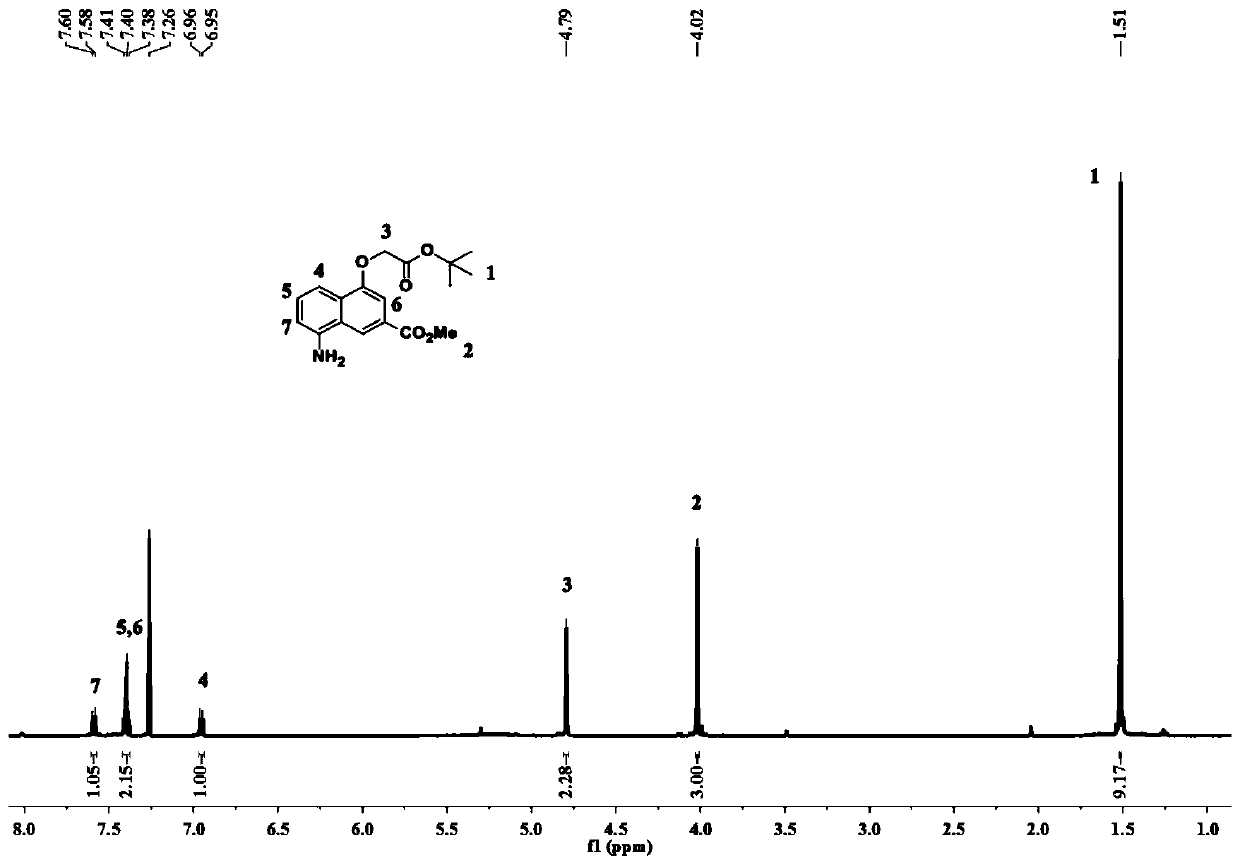
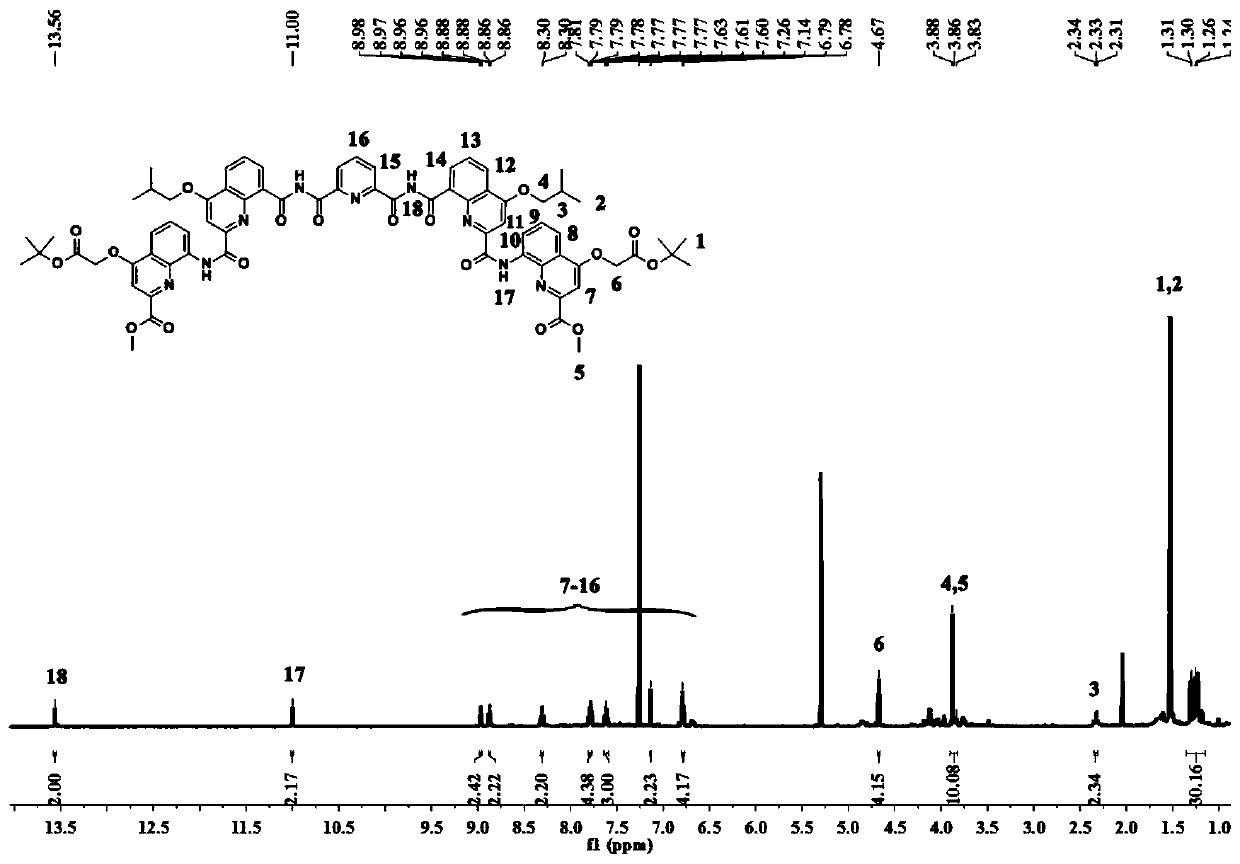
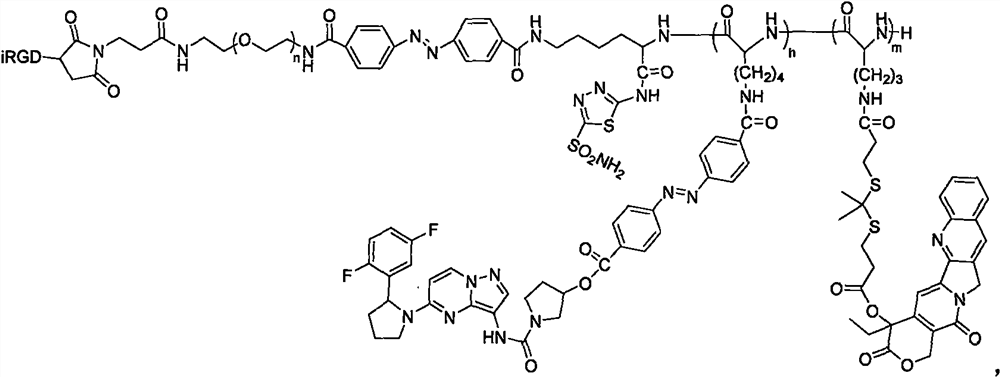
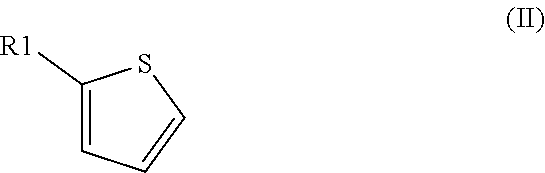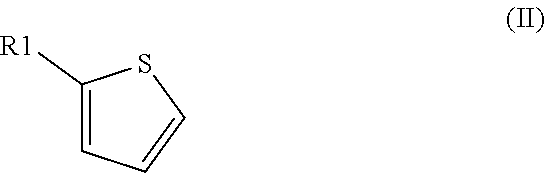Patents
Literature
42 results about "Carbobenzoxy chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
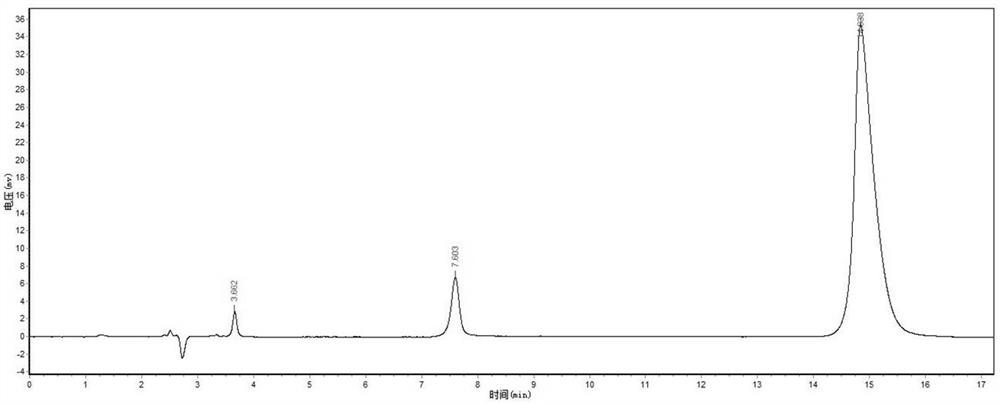
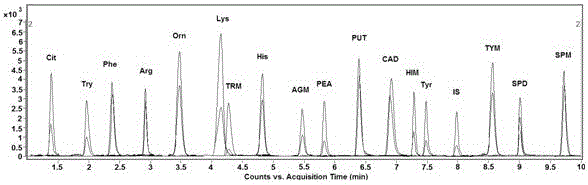
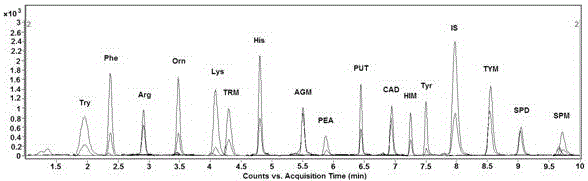
Analysis method for simultaneous detection of amino acids and biogenic amines in foods
ActiveCN105866316AEasy to separateHigh detection sensitivityComponent separationDerivatizationUltrasound assisted
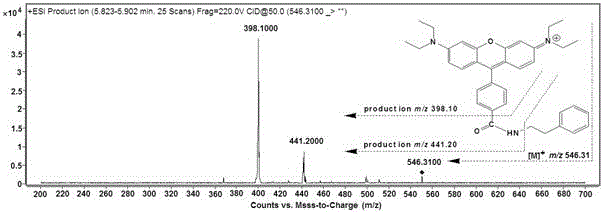
The invention belongs to the field of analytical chemistry and particularly relates to an analysis method for simultaneous detection of amino acids and biogenic amines in foods. The method is characterized in that for the first time, 4'-carbonyl chloride-rhodamine is used as a derivatization reagent to realize simultaneous derivatization of the amino acids and the biogenic amines in the food by means of ultrasonic assistance, in-situ derivation and dispersive liquid-liquid microextraction, and qualitative and quantitative detection and analysis are performed by means of ultra-high performance liquid chromatography coupled with triple quadrupole tandem mass spectrometry in multi-reaction monitoring mode. The method can be used for simultaneous analysis and detection of eight amino acids and nine biogenic amines; by adoption of 1,7-diamino heptane as an internal standard substance, contents of the amino acids and the biogenic amines in different foods can be obtained accurately according to an internal standard method for quantification. The method has advantages of simplicity, quickness, high sensitivity, high selectivity and the like, and high recovery rate is achieved. In addition, by the analysis method, accurate and reliable technical means can be provided for food quality assessment and supervision.
Owner:食药环检验研究院(山东)集团有限公司
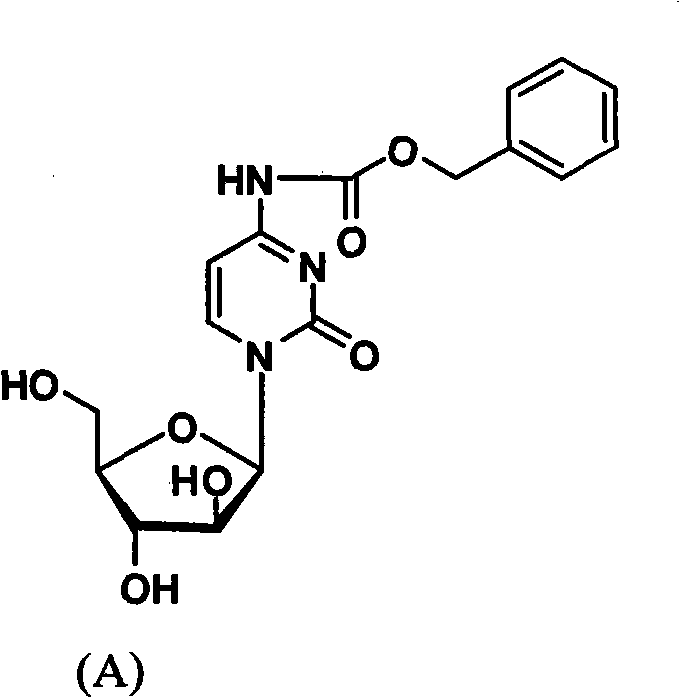
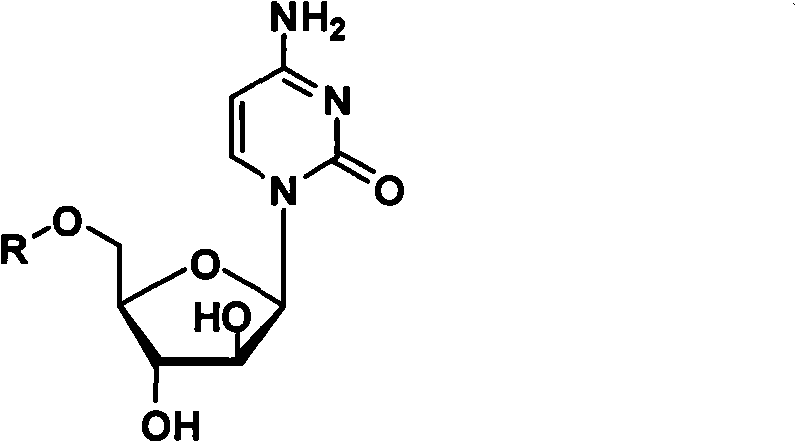
Cytarabine 5'-O-amino-acid ester, salts thereof and preparation method thereof
InactiveCN101812105AGood membrane permeabilityIncrease Absolute BioavailabilityOrganic active ingredientsSugar derivativesCytarabineSodium bicarbonate
The invention belongs to the technical field of medicines and discloses cytarabine 5'-O-amino-acid ester, pharmaceutically acceptable salts thereof and a preparation method thereof. The preparation method comprises the following steps of: slowly dropping carbobenzoxy chloride into a solution formed by cytarabine, sodium bicarbonate and N,N-dimethylacetylamide, and obtaining a compound A after reacting at room temperature; using the compound A and N-butyloxy formoxyl-amino acid as raw materials; adding a reagent to the solution to carry out an esterification reaction to obtain the cytarabine 5'-O-amino-acid ester; and then adding acid to obtain a finished product. The pharmaceutically acceptable salts comprise hydrochlorides, sulfates, formates, acetates, mesylates, propionates, butyrates, p-toluene sulphonates, phosphates, bisulfates, maleates, lactates, carbonates, bicarbonates, malonates, and salts formed with acidic amino acids, and the like. The invention can obviously improve the membrane permeability of the cytarabine so as to improve the bioavailability of the cytarabine.
Owner:SHENYANG PHARMA UNIVERSITY
Dicarba-closo-dodecaborane derivatives
A medicament comprising as an active ingredient a compound or a physiologically acceptable salt thereof represented by general formula (I): wherein R1 represents a dicarba-closo-dodecaboran-yl which may be substituted with a lower alkyl group, a lower alkenyl group, carboxyl group or the like; R2 represents carboxyl group, a lower alkoxycarbonyl group, or hydroxyl group; and X represents a single bond or a linking group such as —CO—Y1— wherein Y1 represents oxygen or —N(R3)— wherein R3 represents hydrogen or a lower alkyl.
Owner:GOLDEN ORCHID BROS
Estrogen amino-acid ester compound with antitumor activity as well as synthetic method thereof
ActiveCN102079771AGood water solubilityGood inhibitory effectSteroidsDipeptidesSodium bicarbonateSolubility
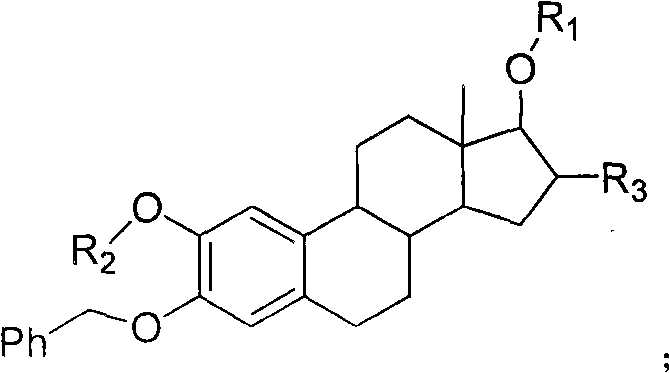
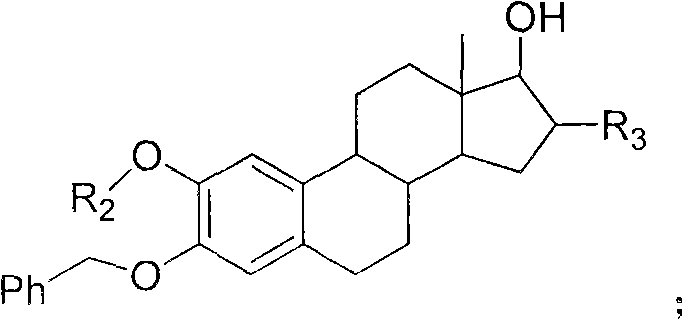
The invention relates to an estrogen amino-acid ester compound with antitumor activity as well as a synthetic method thereof, effectively solving the preparation problems of the estrogen amino-acid ester compound with the antitumor activity. The synthetic method comprises the following steps of: dissolving amino acids or small molecule peptides to an aqueous solution of sodium hydroxide or a saturated solution of sodium bicarbonate, dripping carbobenzoxy chloride, making the solution react until the solution is not purple by developing with ninhydrin or carrying out film detection; washing with aether, removing aether, adjusting the pH value of the water phase with hydrochloric acid until milky white solid occurs and extracting with ethyl acetate; washing mixed solution with distilled water and saturated common salt solution, drying with anhydrous magnesium sulfate, carrying out suction filtration, concentrating and purifying; and then, mixing the mixed solution with sterides nucleus and dissolving the mixture to a reaction solvent, adding a condensing agent and a catalyst, supplementing an additional condensing agent, carrying out film detection, filtering, concentrating and purifying, dissolving in a dissolving solvent, adding palladium carbon for catalytic hydrogenation, detecting the film, filtering, concentrating and purifying. The estrogen amino-acid ester compound withantitumor activity, provided by the invention, has favorable water solubility and is superior to 2-methoxyestradiol in the tumor cell resistance action.
Owner:ZHENGZHOU UNIV
Preparation method and applications of oriented immobilized PEGA composite resin
ActiveCN104844710ADoes not affect conformationDoes not affect activityCarrier-bound/immobilised peptides4-nitrobenzyl alcoholTert-Butyloxycarbonyl protecting group
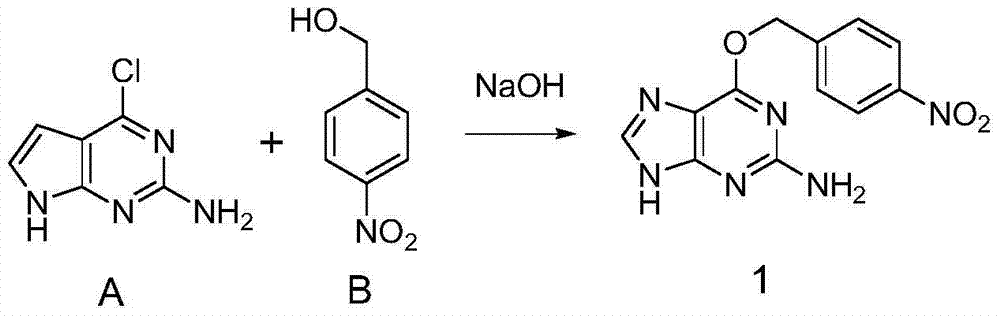
The invention discloses a preparation method and applications of an oriented immobilized PEGA composite resin. The preparation method comprises following steps: 2-amino-6-chloropurine and 4-nitrobenzyl alcohol are taken as raw materials for Williamson ether synthesis so as to obtain 4-nitryl-O6-benzylguanine; 4-nitryl- O6-benzylguanine is subjected to reduction reaction with protection of t-butyloxycarboryl; coupling reaction of an obtained compound with gamma-aminobutyric acid protected by carbobenzoxy chloride is carried out in the presence of ethyl dimethyl carbodiimide and 1-hydroxybenzotriazole; O<6>-benzylguanine derivative modified PEGA resin is obtained via reduction, separation and purification, reaction with PEGA, and deprotection; and transalkylation reaction of the O<6>-benzylguanine derivative modified PEGA resin with a protein containing MGMT label is carried out, so that oriented immobilization on PEGA resin surface via thioether covalent bonds is realized. Reaction of the preparation method is stable; the steps are simple; a stable uniform protein coating with uniform orientation can be formed on solid material surface by a prepared product; and the preparation method is used for preparing reagent kit with specific recognition effects.
Owner:NORTHWEST UNIV
Novel near infrared ratio fluorescence probe for detecting hydrogen peroxide as well as preparation method and application of probe
InactiveCN109096319AHigh selectivityEasy to synthesizeGroup 3/13 element organic compoundsFluorescence/phosphorescenceFluorescenceIodide
The invention discloses a novel near infrared ratio fluorescence probe for detecting hydrogen peroxide as well as a preparation method and an application of the probe. The probe is 2-[2-[2-[[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyloxycarbonyl]benzylamino]-3-[(1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene)ethylidene]-1-cyclohexen-1-yl]ethenyl]-1,3,3-trimethylindolium iodide. The preparation method comprises steps as follows: 1,2,3,3-tetramethyl-3H-iodoindole and 2-chloro-1-formyl-3-hydroxymethylcyclohexene are subjected to a dehydration condensation reaction; obtained 2-[2-[2-chloro-3-[(1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene)ethylidene]-1-cyclohexen-1-yl]ethenyl]-1,3,3-trimethylindolium iodide and benzylamine react; obtained 2-[2-[2-benzylamino-3-[(1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene)ethylidene]-1-cyclohexen-1-yl]ethenyl]-1,3,3-trimethylindolium iodide and 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)carbobenzoxy chloride react, and the novel fluorescence probe is obtained. The probe shows higher selectivity for detection of hydrogen peroxide.
Owner:HEZHOU UNIV
Method to purify and stabilize chloroolefins
The present invention is directed towards a method of purification of chloroolefins having 3 carbons and to a method to provide stable compositions of chloroolefins having 3 carbons. The chloroolefins are purified via the use of a solid adsorbents for the removal of decomposition product such as phosgene and / or phosgene precursory from the reaction of the chloroolefins with oxygen. The chloroolefins stabilized against an increase of the phosgene level via the addition of inhibitors to the chloroolefins.
Owner:ARKEMA FRANCE SA
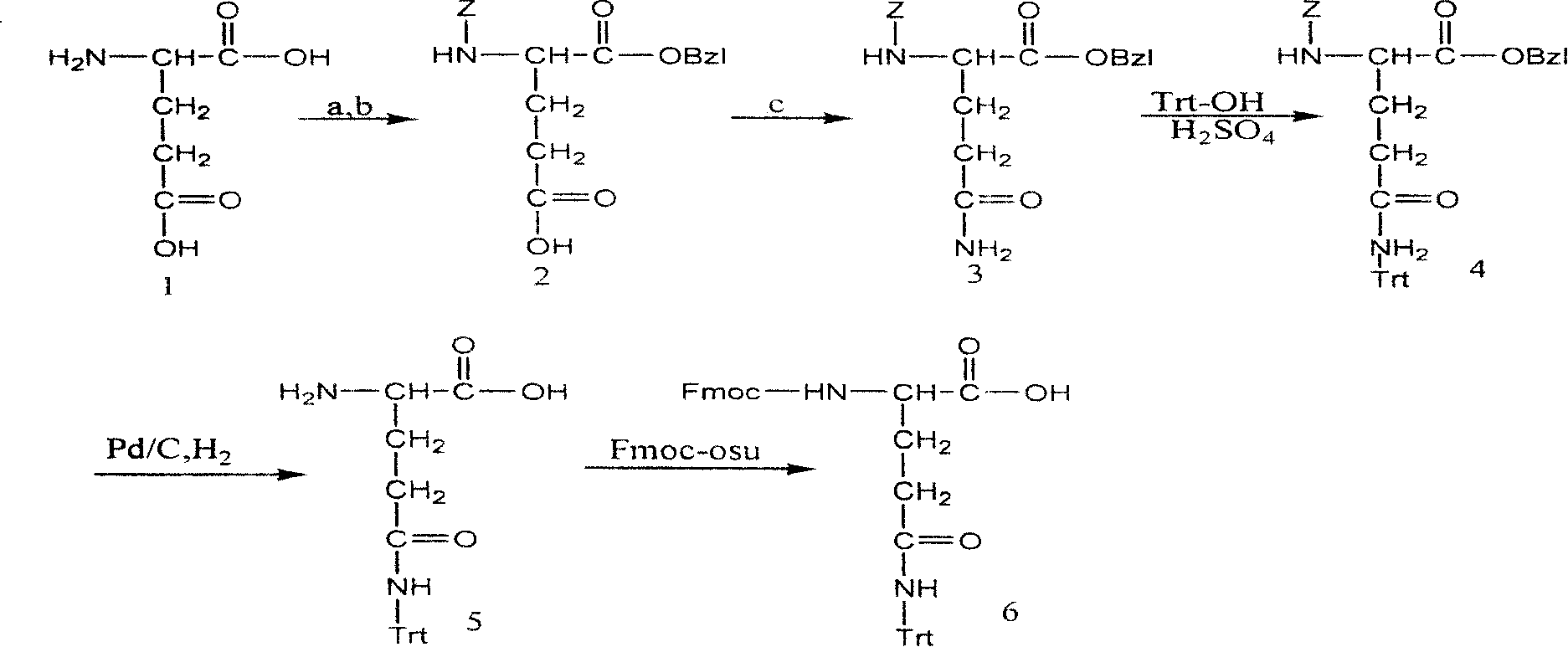
Method for synthesizing N-fluorene methoxycarbonyl-N-trityl-D-glutamine
ActiveCN101219970AReduce manufacturing costCarbamic acid derivatives preparationOrganic compound preparationEthyl chloroformateN dimethylformamide
The invention relates to a synthetic method of N-fluorenylmethoxycarbonyl-N- triphenylmethyl-D-glutamine to solve the current problems of the shortage of D-Gln raw materials and high cost of synthesizing products from the D-Gln. The synthesis includes the following steps: a. D-glutamate and a carbobenzoxy chloride are reacted to get the N-carbobenzoxy-D-glutamate; in an organic solvent N and N-dimethylformamide with the existence of a triethylamine and bromomethyl-benzene, the N-carbobenzoxy-D-glutamate selectively protects an Alpha-carboxyl to get an N-benzyloxycarbonyl-D-benzyl L-glutamate; b. the triethylamine, ethyl chloroformate and ammonia are added to N- benzyloxycarbonyl-D- benzyl L-glutamate organic solvent with a molar ratio of 1:1:2 to 6; after reacted for 6 to 24 hours under a temperature of -20 to 20 DEG C, an N- benzyloxycarbonyl-D-glutaminebenzylester is gotten; c. the product of b, acetate liquor is reacted with a triphenylmethanol under a catalysis of concentrated sulfuric acid, and N- benzyloxycarbonyl-N-triphenylmethyl-D-glutaminebenzylester can be gotten after reacted for 8 to 24 hours under a temperature of 40 to 60 DEG C; d. benzyloxycarbonyl and benzyl are detracted from the product of c to get the N-triphenylmethyl-D-glutamine; e. the product of d is protected by an Fmoc group to get the N-fluorenylmethoxycarbonyl-N-triphenylmethyl-D- glutamine.
Owner:GL BIOCHEM SHANGHAI
Polyimide-polybenzoxazole precursor solution, polyimide-polybenzoxazole film, and preparation method therefor
This invention relates to a polyimide-polybenzoxazole precursor solution, a polyimide-polybenzoxazole film, and a method of manufacturing the same, wherein a film manufactured using the polyimide-polybenzoxazole precursor solution of the invention is formed by copolymerizing a unit structure of diamine and dianhydride and a unit structure of diaminophenol and dicarbonyl chloride in an organic solvent, and is colorless and transparent, like conventional polyimide films, and can exhibit improved heat resistance and low birefringence.
Owner:KOLON IND INC
Preparation method of N-carbobenzoxy-3-amino-alanine tert-butyl ester
InactiveCN109535035AIncreased overall process yieldReduce manufacturing costCarbamic acid derivatives preparationOrganic compound preparationN dimethylformamideBenzyl chloroformate
The invention discloses a preparation method of tert-butyl N-carbobenzoxy-3-amino-alanine tert-butyl ester, and mainly solves the technical problem of low total yield and high cost in the original process. The preparation method comprises the following steps of 1, suspending asparagine into a mixed solution of water and acetone; regulating the pH value to a basic state by a sodium carbonate solution; lowering the temperature; adding carbobenzoxy chloride; then, raising the temperature for reaction; after the reaction is finished, performing acidification by hydrochloric acid; separating out solid; performing filtering to obtain an intermediate N-benzoxycarbonyl asparagine; 2, suspending the N-benzoxycarbonyl asparagine into dichloromethane; introducing isobutene; adding concentrated sulfuric acid; performing sealed reaction for two days; performing treatment to obtain N-carbobenzoxy asparagine tert-butyl ester; 3, dissolving the N-carbobenzoxy asparagine tert-butyl ester into water andN,N-dimethylformamide; adding iodobenzene diacetate for reaction first; then adding pyridine; continuously performing reaction till the completion; performing treatment to obtain a final product.
Owner:GL BIOCHEM SHANGHAI +1
Methods for producing nateglinide crystals
InactiveUS20070167523A1Easy procedureEasily filtered outBiocideOrganic active ingredientsIsopropylKetone solvents
There is provided methods for producing nateglinide crystals, which comprises the steps of adding an acid(s) to a reaction mixture containing nateglinide to make it acidic, the reaction mixture being obtained by reacting trans-4-isopropylcyclohexylcarbonyl chloride with D-phenylalanine in a mixed solvent of ketone solvent and water in the presence of an alkali; and then adjusting the temperature of the mixture to 58° C. to 72° C. and the concentration of ketone solvent to more than 8 wt % and less than 22 wt % to conduct precipitation of nateglinide crystals. This producing method is the industrially beneficial methods for crystallization of nateglinide.
Owner:AJINOMOTO CO INC
Recyclable catalysts for chlorination of organic acids and alcohols
ActiveUS20170274362A1Easy to separateWithout losing efficiencyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsRecyclable catalystOrganic acid
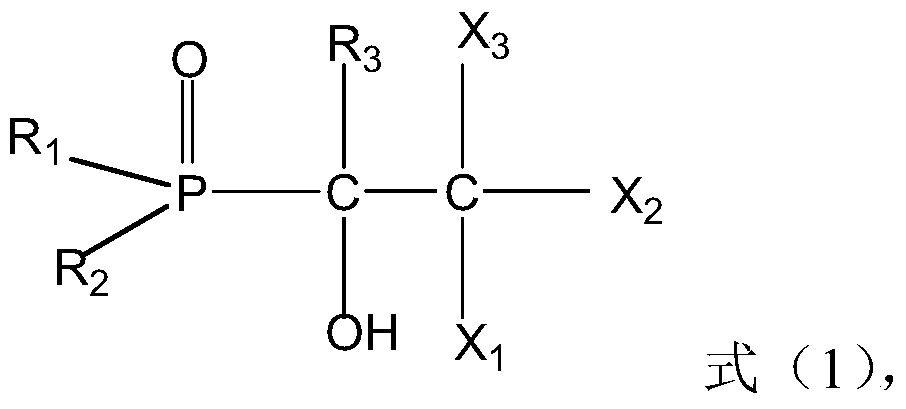
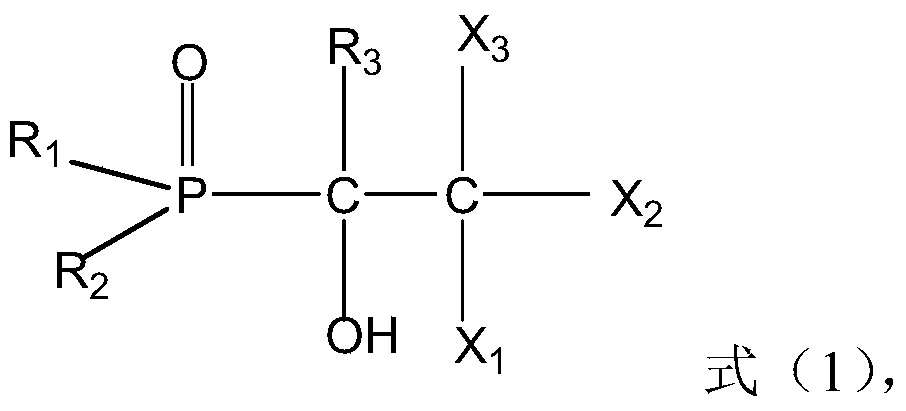
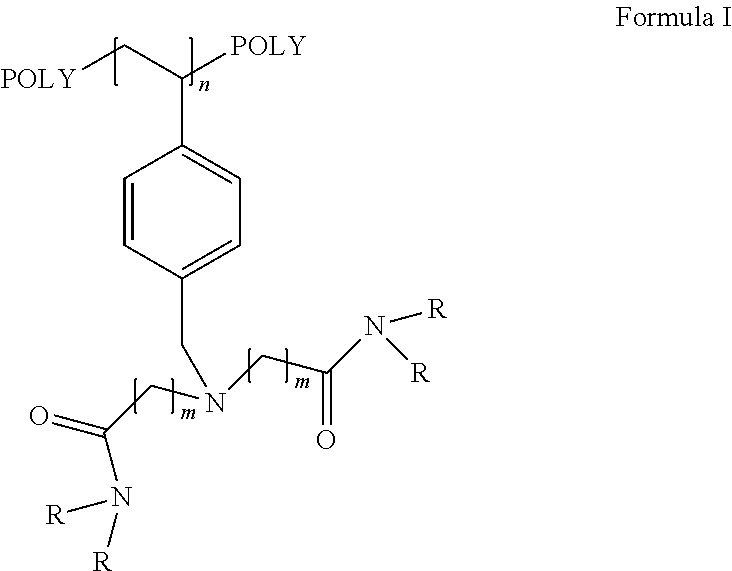
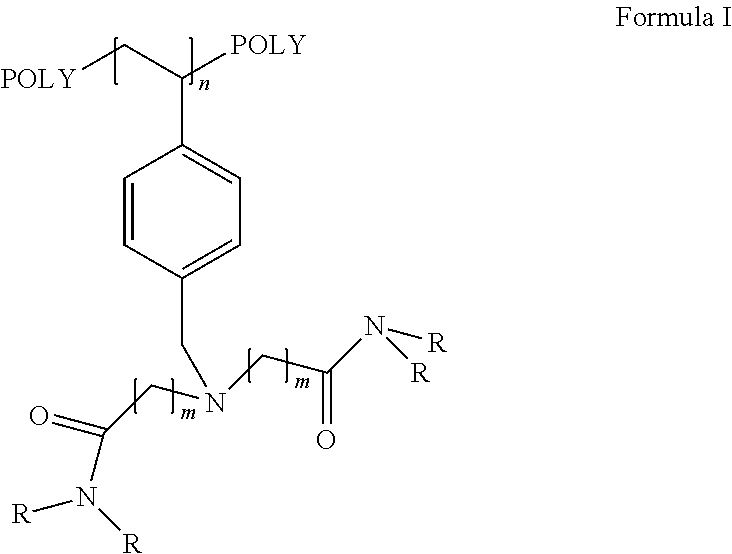
The present invention discloses recyclable polymeric catalyst of Formula I, for chlorination of organic acids and alcohols using chlorinating agents such as carbonyl chloride, oxalyl chloride or thionyl chloride,wherein, ‘m’ on the pendent groups on polystyrene backbone can have values from 1 to 5 and R is the alkyl group ranging from C1 to C5.
Owner:GALAXY SURFACTANTS
Method for preparing polycarbonate
The invention relates to a method for preparing polycarbonate. The method utilizes an interface method, and comprises the steps of: mixing alkaline aqueous solution of one or more dihydroxy aromatic hydrocarbons and organic solution of carbonyl chloride(phosgene); controlling the pH value to be between 9 and 12.5 in the whole process; and performing an interfacial polymerization reaction of a phenol chain terminator, the dihydroxy aromatic hydrocarbons and the phosgene in the presence of a catalyst, namely an amphiphilic block copolymer to prepare the polycarbonate. The method utilizes the amphiphilic block copolymer as the catalyst for preparing the polycarbonate by the interface method, which can increase the utilization rate of the carbonyl chloride, reduce the consumption of the carbonyl chloride, and improve the utilization rate of bisphenol; the catalyst can promote the rapid transformation of chloroformate and obtain a polycarbonate product with low inclusion amount of dihydroxy aromatic compounds; and no phenol precipitations are precipitated after a wastewater phase is acidified, and a catalyst material can be treated and recovered by adopting a conventional method.
Owner:CHINA NAT BLUESTAR GRP CO LTD
Alcaftadine intermediate and synthetic method for alcaftadine
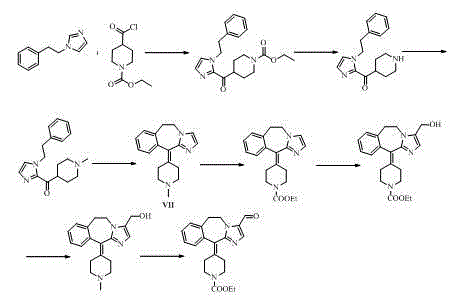
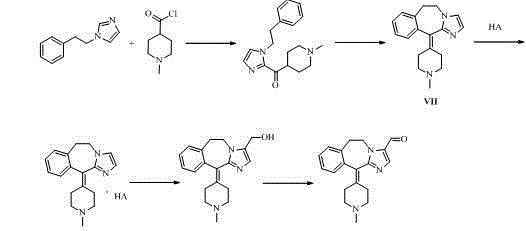
ActiveCN104860888AAvoid it happening againHigh synthetic yieldGroup 4/14 element organic compoundsEthyl groupMethyl palmoxirate
The invention belongs to the field of medicinal chemistry and particularly relates to an alcaftadine intermediate and a synthetic method for alcaftadine. The method comprises the following steps: taking (1H-imidazo-5-yl)methanol (as shown in a formula II) as a raw material, and producing a hydroxyl protection reaction, a substitution reaction with beta-phenylethyl bromide, an electrophilic substitution with 1-methylpiperidine-4-carbonyl chloride hydrochloride, a cyclization reaction and an oxidation reaction to synthesize alcaftadine (as shown in a formula I). The method provided by the invention does not relate to an imidazole ring hydroxymethylation reaction, and hydroxymethyl in the alcaftadine structure is introduced from a starting material, so that the use of high-temperature condition and a toxic reagent, namely formaldehyde is reduced, the production of bis(hydroxymethyl) byproducts in a hydroxymethylation process is avoided, and the cost is reduced. The alcaftadine intermediate is low in production cost, good in product quality and suitable for industrialized production, and the synthetic raw materials are low in price and easily available.
Owner:NANJING HUAWE MEDICINE TECH DEV
Polylactic acid copolymer with high glass-transition temperature and preparation method thereof
The invention discloses a polylactic acid copolymer with high glass-transition temperature and a preparation method thereof. Lactide and copolymerization monomer as main raw materials are subjected to ring opening polymerization and polycondensation reactions in the presence of a catalyst, so as to prepare the polylactic acid copolymer. The copolymer contains an aromatic diacid monomer unit and a diol monomer unit with ring structure, has weight average molecular weight of 3-300000 and the glass-transition temperature of 110-200 DEG C. The preparation method of the invention is simple, the raw material is lactide rather than a lactic acid solution, and the aromatic dicarboxylic acid rather than non phosgene or oxalic acid is used. By adjusting the ratio of materials for ring opening polymerization, can be simple, polylactic acid copolymers with different monomer unit ratio can be precisely designed, and the prepared polylactic acid copolymer has high molecular weight and glass transition temperature up to 200 DEG C.
Owner:JIANGSU SUPLA BIOPLASTICS CO LTD
Purification method of isocyanate silane coupling agent and isocyanate silane coupling agent
PendingCN112062786ASimple technical routeImprove applicabilitySilicon organic compoundsSulfonyl chlorideEngineering
The invention discloses a purification method of an isocyanate silane coupling agent and the isocyanate silane coupling agent, alkyl carbonyl chloride or alkyl sulfonyl chloride is added into a crudeproduct containing the isocyanate silane coupling agent in a nitrogen atmosphere, after full mixing, vacuum distillation is carried out, and the isocyanate silane coupling agent is obtained. The method disclosed by the invention has the beneficial effects that the method is suitable for purifying the isocyanate silane coupling agent prepared by adopting a thermal cracking method, the raw materialsare cheap and easy to obtain, the addition amount is small, the process is simple, the manufacturing cost is hardly increased, and side reactions such as bulk polymerization and the like generated when the isocyanate silane coupling agent is rectified and heated can be effectively inhibited.
Owner:ZHANGJIAGANG HUASHENG CHEM CO LTD
Synthesis process of di-tert-butyl dicarbonate
ActiveCN112521285AImprove responseRaise the reaction temperaturePreparation from organic carbonatesBulk chemical productionCarbonyl chlorideReaction temperature
The invention discloses a synthesis process of di-tert-butyl dicarbonate. According to the synthesis process, tert-butyl alcohol alkali metal salt and a carbonyl chloride compound are used as reactionraw materials; supercritical carbon dioxide is used as a reaction raw material and a solvent; and synthesis of the di-tert-butyl dicarbonate is carried out in a carbon dioxide supercritical state; the problem that no proper solvent exists in each step of reaction for producing di-tert-butyl dicarbonate is thoroughly solved by utilizing the super-strong solubility and permeability of the supercritical carbon dioxide, the carbon dioxide is used as both the reaction raw material and reaction solvent, so that the reaction is easier, the reaction temperature is increased to room temperature or above, and no side reaction exists; the supercritical carbon dioxide has different solubilities for a product and a by-product, so that the product is directly separated from the by-product, and the problems of post-treatment water pollution and product loss are avoided.
Owner:山东习尚喜新材料科技股份有限公司
Synthesis method of racemic-(3aR,7aR)-5-(t-butyloxycarboryl)-octahydro furan [3,2-c] pyridine-3a- carboxylic acid
InactiveCN107383034AReasonable reaction process designMethod route shortOrganic chemistry methodsFuranLithium hydroxide
The invention relates to a synthesis method of racemic-(3aR,7aR)-5-(t-butyloxycarboryl)-octahydro furan [3,2-c] pyridine-3a-carboxylic acid. The method mainly solves the technical problem that no proper industrial synthesis method exists in the prior art. The method comprises seven steps that: firstly, 2-bromoethanol and ethyl propiolate react in a solvent of methylene dichloride at the room temperature to obtain a compound 2; then, the compound 2 and ethyl cyanoacetate react in a solvent of N, N-dimethyl formamide at the room temperature over the night to obtain a compound 3; the compound 3 is subjected to catalytic hydrogenation to reduce the cyano groups to obtain amino groups; meanwhile, the exchange with the self ester is performed to obtain a compound 4; a compound 5 is obtained through carbobenzoxy chloride on the compound 4; the compound 5 is reduced by borane to obtain a compound 6; finally, the compound 6 uses a palladium hydroxide as a catalyst in ethanol; meanwhile, Boc estolide is added; hydrogen gas is introduced for overnight reaction to obtain a target compound 7; the compound 7 is hydrolyzed by water and lithium hydroxide in a mixed solution of methanol and water; a final target compound 8 is obtained.
Owner:上海药明康德新药开发有限公司 +4
Synthesis method of combined buprofezin
PendingCN113651771AImprove qualityEasy to manufactureCarbamic acid derivatives preparationOrganic compound preparationMethylanilineBenzoyl peroxide
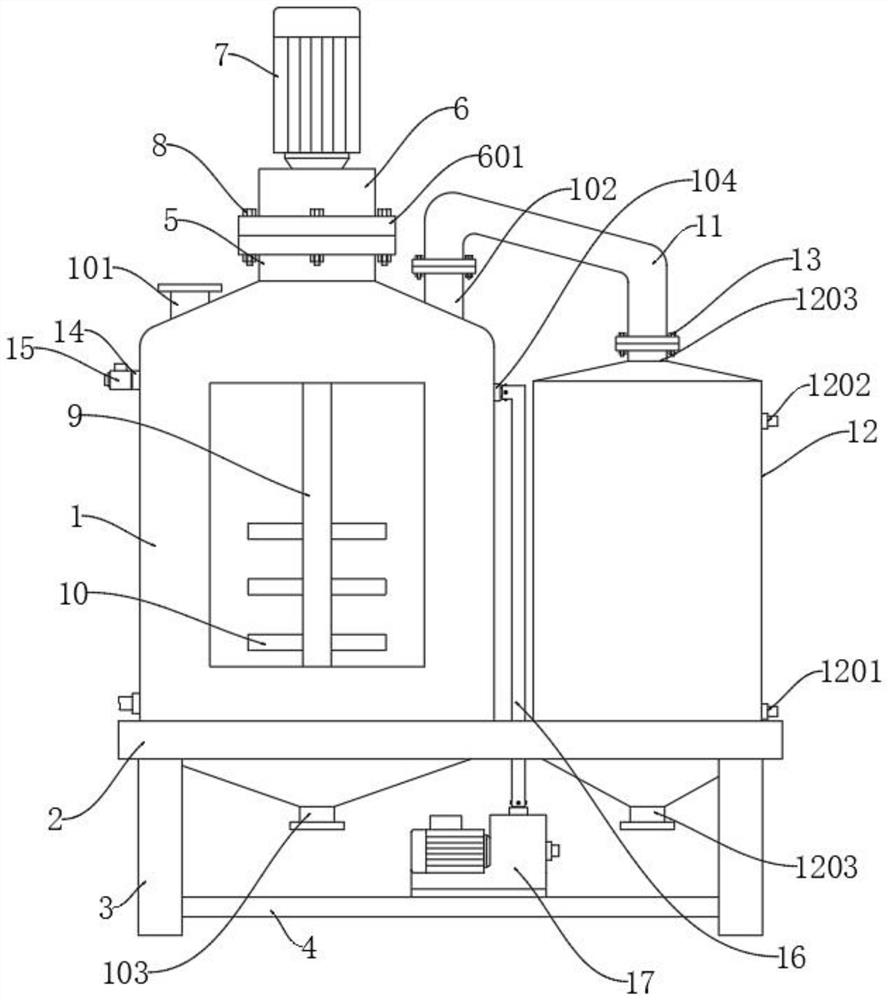
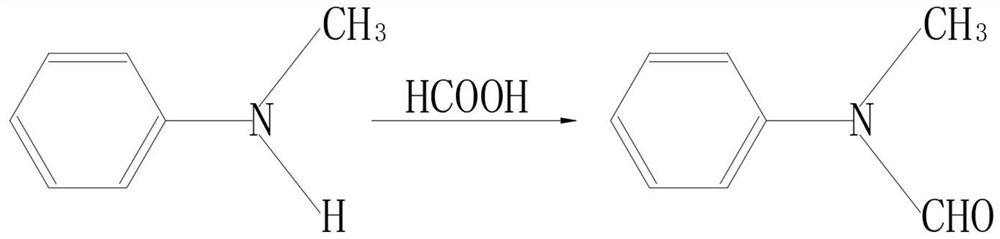
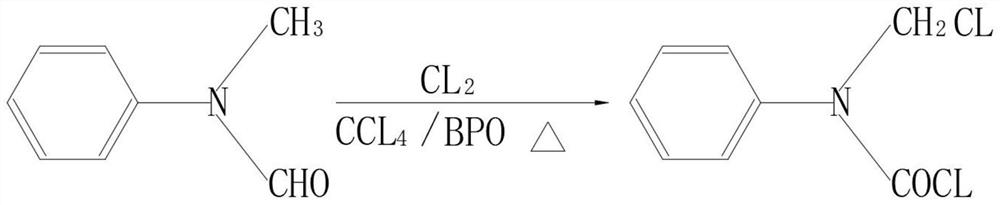
The invention discloses a synthesis method of combined buprofezin, relates to the technical field of synthesis methods of buprofezin, and aims to solve the problem that carbonyl chloride crop organic intermediates are usually used for reaction in the existing synthesis process of combined buprofezin, but carbonyl chloride is a violent suffocating poison gas and can cause harm to human bodies after being inhaled by people. The synthesis method comprises the following steps of: adding N-methylaniline and formic acid into a reaction kettle to prepare N-methyl formyl amine, adding the prepared N-methyl formyl amine, carbon tetrachloride and benzoyl peroxide into a distillation kettle, then introducing chlorine gas to prepare an N-chloromethyl-N-phenyl carbamoyl chloride solution, then preparing tert-butyl isothiocyanate preparing an N-tert-butyl-N'-isopropyl thiourea solution, and adding the prepared N-chloromethyl-N-phenyl carbamoyl chloride solution, tert-butyl isothiocyanate, acetone, dimethyl formamide and potassium hydroxide into a reaction kettle for reaction to obtain buprofezin crystals.
Owner:ANHUI GUANGXIN AGROCHEM
Aromatic helical foldamer as well as preparation method and application of aromatic helical foldamer
InactiveCN109705098AImprove solubilityStrong π-π stacking effectOrganic chemistrySynthesis methodsQuinoline
The invention discloses an aromatic helical foldamer as well as a preparation method and application of the aromatic helical foldamer. The aromatic helical foldamer provided by the invention is dimethyl8,8'-((8,8'-(((pyridine-2,6-dicarbonyl)bis(aza-2-yl))bis(carbonyl))bis(4-isobutoxyquinoline-2,2'-carbonyl))bis(nitroalkyl-2-yl))bis(4-(2-(tert-butoxy)-2-oxyethoxy)quinoline-2-tert-butyl carboxylate.A synthesis method comprises the steps: firstly, preparing 8,8'-((pyridine-2,6-dicarbonyl)bis(aza-2-yl))bis(carbonyl))bis(4-isobutoxyquinoline-2-carbonyl chloride, and then, preparing the target aromatic helical foldamer. At present, a helical structure shows an unprecedented potential in the fields such as chiral separation, molecular recognition and display material, the aromatic helical foldamer is successfully synthesized, and novel thoughts and approaches are provided for the construction of an aromatic helical structure.
Owner:JILIN NORMAL UNIV
Hypoxic/ROS response type prodrug for blocking pancreatic duct adenocarcinoma innervation through deep penetration
ActiveCN114272247AOrganic active ingredientsPeptide preparation methodsPropanoic acidPolyethylene glycol
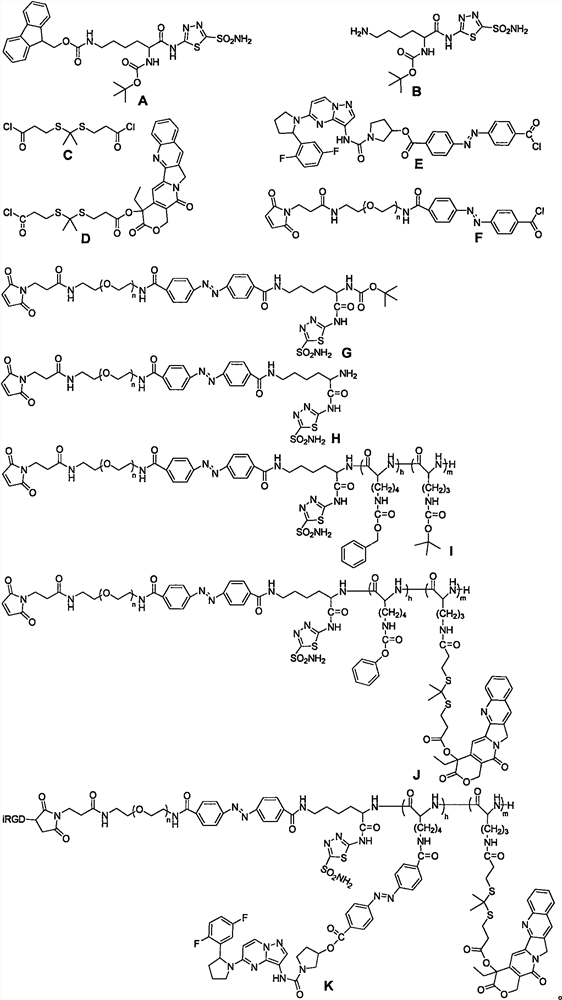
The invention relates to a hypoxic / ROS (reactive oxygen species) responsive prodrug for deeply penetrating and blocking pancreatic duct adenocarcinoma innervation and a preparation method of the prodrug. The preparation method comprises the following steps: (1) reacting N-alpha-fluorenylmethoxycarbonyl-N-epsilon-t-butyloxycarbonyl-L-lysine with 5-amino-1, 3, 4-thiadiazole-2-sulfonamide to obtain (A); reacting (A) with piperidine to obtain (B); (2) dimethyl dithiopropionic acid methane and thionyl chloride are subjected to a reaction, and (C) is obtained; reacting the compound (C) with camptothecin to obtain a compound (D); (3) azobenzene-4, 4-dicarbonyl chloride and lalotinib are subjected to a reaction, and (E) is obtained; (4) reacting maleimide polyethylene glycol with azobenzene-4, 4-dicarbonyl chloride to obtain (F); (F) reacts with (B) to obtain (G); reacting (G) with trifluoroacetic acid to obtain (H); the reaction product (H) sequentially reacts with N6-carbobenzoxy-L-lysine cyclic anhydride and N5-t-butyloxycarboryl-L-ornithine cyclic anhydride, and (I) is obtained; reacting (I) with trifluoroacetic acid, adding (D), and reacting to obtain (J); reacting (J) with hydrobromic acid and acetic acid, adding (F) and iRGD, and reacting to obtain a polymer prodrug (K); the invention also discloses a method for preparing a nano-drug from the polymer prodrug.
Owner:NINGBO UNIV
A kind of biomass-based slow-release pesticide and preparation method thereof
InactiveCN106508898BIncrease profitIncrease added valueBiocideAnimal repellantsPtru catalystOrganosolv
The invention discloses a biomass-based slow-release pesticide and a preparation method thereof. The method comprises the following steps: (1) carrying out a first contact reaction on a biomass raw material, fatty alcohol and a first acidic catalyst, and separating the product obtained after the first contact reaction so as to obtain light oil and alcoholysis residue; and (2) carrying out a third contact reaction on the alcoholysis residue and a pesticide drug compound and an optional cross-linking agent in an organic solvent at the pH value of 7-9 and at 30-50 DEG C for 0.5-1 h. The fatty alcohol is C4-C13 aliphatic saturated monohydric alcohol. The cross-linking agent is one or more ingredients selected from a group consisting of carbonyl chloride, thionyl chloride, isocyanate, maleic anhydride and phthalic anhydride. Drug loading capacity of the prepared biomass-based slow-release pesticide reaches up to 25.47 wt%. The pesticide has high insect poisoning efficiency. After three months of the use, the highest inhibition rate of fruit fly eggs still reaches up to 80% and above.
Owner:SHENYANG RES INST OF CHEM IND +1
A kind of preparation method of amino acid surfactant
ActiveCN109482100BHigh yieldNo purification requiredOrganic compound preparationTransportation and packagingActive agentSurface-active agents
The invention discloses a preparation method of an amino acid surfactant. The specific steps are as follows: (1) at room temperature, add a fatty acid and a catalyst into a four-necked flask, slowly raise the temperature to 50-70 degrees, drop thionyl chloride or pass through Add carbonyl chloride and react for 3-6 hours to obtain fatty acid chloride; (2) at room temperature, add amino acid and caustic alkali solution in a four-necked flask, control the pH range to 10-12, and the temperature is 10-20 degrees, and dropwise Fatty acid chloride and caustic alkali solution, keep the pH constant during the dropwise addition, react for 3-6 hours, and obtain the crude product of amino acid surfactant; (3) add inorganic acid to adjust the pH to 1-2, then add cold water, filter and dry to obtain The final amino acid surfactant product. The invention has low production cost, high efficiency and simple process, is suitable for industrialized production, and under the premise of ensuring high yield and excellent product appearance, the solvent is recovered, which is green and environment-friendly.
Owner:SHENZHEN HENGWEIXIANG SCI & TECH CO LTD
Method for producing N-benzyloxy carbonyl glutamic acid
InactiveCN1318396CGood for clean removalAvoid Adverse Conditions That Affect Full ExtractionCarbamic acid derivatives preparationOrganic compound preparationOrganic solventBenzyl chloroformate
The present invention relates to the production process of N-benzyloxy carbonyl glutamic acid, and belongs to the field of medicine chemical technology. The production process includes the reaction of glutamic acid and carbobenzoxy chloride under alkali condition to produce N-benzyloxy carbonyl sodium glutamate solution with impurity; extraction to eliminate imipurity; and processing the solution to obtain the product. The production process features that the N-benzyloxy carbonyl sodium glutamate solution with impurity is processed through pH regulation to 3-4 with acid, organic solvent extraction of impurity, pH regulation to strong acidity for separating coarse product and re-crystallization of the coarse product to obtain product. The production process is simple, low in cost, high in product quality and suitable for industrial production.
Owner:四川三高生化股份有限公司
Estrogen amino-acid ester compound with antitumor activity as well as synthetic method thereof
ActiveCN102079771BGood water solubilityEnhanced inhibitory effectSteroidsDipeptidesSolubilitySodium bicarbonate
The invention relates to an estrogen amino-acid ester compound with antitumor activity as well as a synthetic method thereof, effectively solving the preparation problems of the estrogen amino-acid ester compound with the antitumor activity. The synthetic method comprises the following steps of: dissolving amino acids or small molecule peptides to an aqueous solution of sodium hydroxide or a saturated solution of sodium bicarbonate, dripping carbobenzoxy chloride, making the solution react until the solution is not purple by developing with ninhydrin or carrying out film detection; washing with aether, removing aether, adjusting the pH value of the water phase with hydrochloric acid until milky white solid occurs and extracting with ethyl acetate; washing mixed solution with distilled water and saturated common salt solution, drying with anhydrous magnesium sulfate, carrying out suction filtration, concentrating and purifying; and then, mixing the mixed solution with sterides nucleus and dissolving the mixture to a reaction solvent, adding a condensing agent and a catalyst, supplementing an additional condensing agent, carrying out film detection, filtering, concentrating and purifying, dissolving in a dissolving solvent, adding palladium carbon for catalytic hydrogenation, detecting the film, filtering, concentrating and purifying. The estrogen amino-acid ester compound withantitumor activity, provided by the invention, has favorable water solubility and is superior to 2-methoxyestradiol in the tumor cell resistance action.
Owner:ZHENGZHOU UNIV
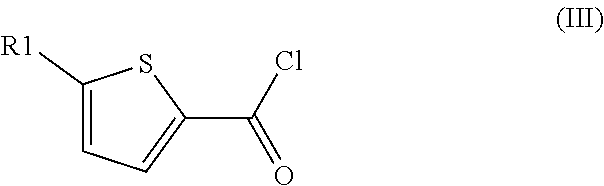
Method for preparation of thiophene-2-carbonyl chlorides with oxalyl chloride
The invention discloses a method for the preparation of thiophene-2-carbonyl chlorides starting from thiophenes with oxalyl chloride at elevated temperature with short reaction time.
Owner:LONZA LTD
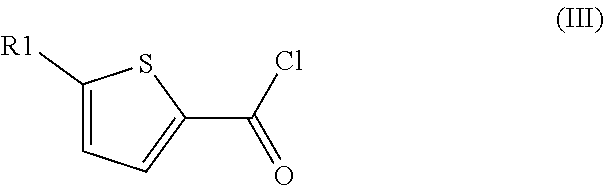
Method for preparation of thiophene-2-carbonyl chlorides with oxalyl chloride
The invention discloses a method for the preparation of thiophene-2-carbonyl chlorides starting from thiophenes with oxalyl chloride at elevated temperature with short reaction time.
Owner:LONZA LTD
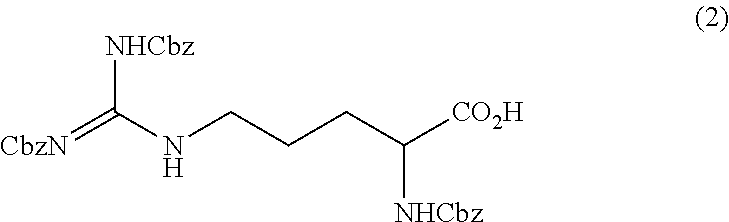
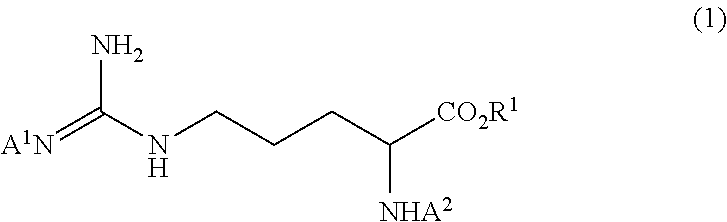
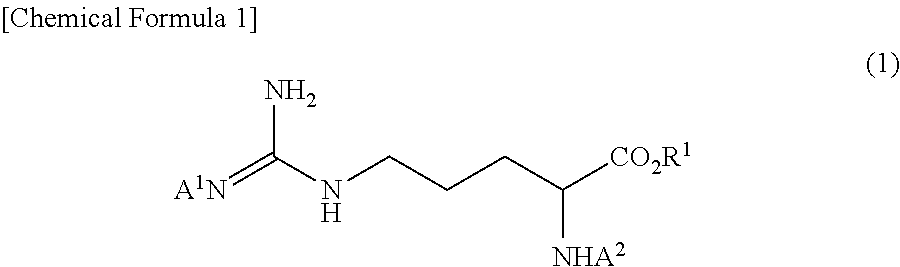
Method for producing tri-carbobenzoxy-arginine
ActiveUS10202337B2Satisfactory fluidityEfficient productionOrganic chemistryOrganic compound preparationOrganic solventArginine
A tri-carbobenzoxy-arginine represented by the following formula (2):is produced by carbobenzoxylating the arginine or arginine derivative (1) represented by the following formula (1), or a salt thereof:by adding carbobenzoxy chloride and a base in a water / organic solvent bilayer system to an arginine or arginine derivative (1) represented by the formula (1), or a salt thereof.
Owner:KANEKA CORP
Preparation method of mesosulfuron-methyl
The invention discloses a mesosulfuron-methyl preparation method, which comprises the following steps: reacting pyrilamine with a carbonyl chloride compound to obtain isocyanate, and condensing the isocyanate with 5-methylsulfonylaminomethyl-2-methoxycarbonyl benzenesulfonamide to obtain mesosulfuron-methyl. The method disclosed by the invention is high in yield, the prepared product is high in purity, and byproducts generated in the preparation process are few and are easy to recover.
Owner:JIANGSU REPONT PESTICIDE FACTORY
A kind of synthesis technique of creatine ethyl ester hydrochloride
ActiveCN108929249BHigh yieldQuick responseOrganic compound preparationOrganic chemistry methodsPtru catalystHigh creatine
The invention provides a new synthesis process of creatine ethyl ester hydrochloride, the synthesis steps are as follows: using creatine in absolute ethanol, using 4-dimethylaminopyridine (DMAP) as a catalyst, thionyl chloride or carbon Acyl chloride is a reactive dehydrating agent, which can be reacted at low temperature to produce creatine ethyl ester hydrochloride. The catalyst effectively improves the esterification reaction speed between creatine and ethanol, reduces the occurrence of creatinine side reactions, and improves the purity of creatine ethyl ester hydrochloride. The overall process basically belongs to normal temperature and pressure process, with low energy consumption, high creatine conversion rate and high product purity.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
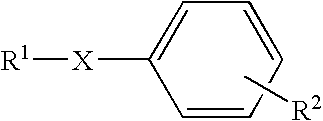
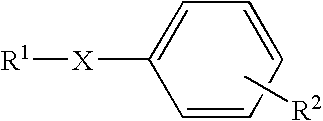
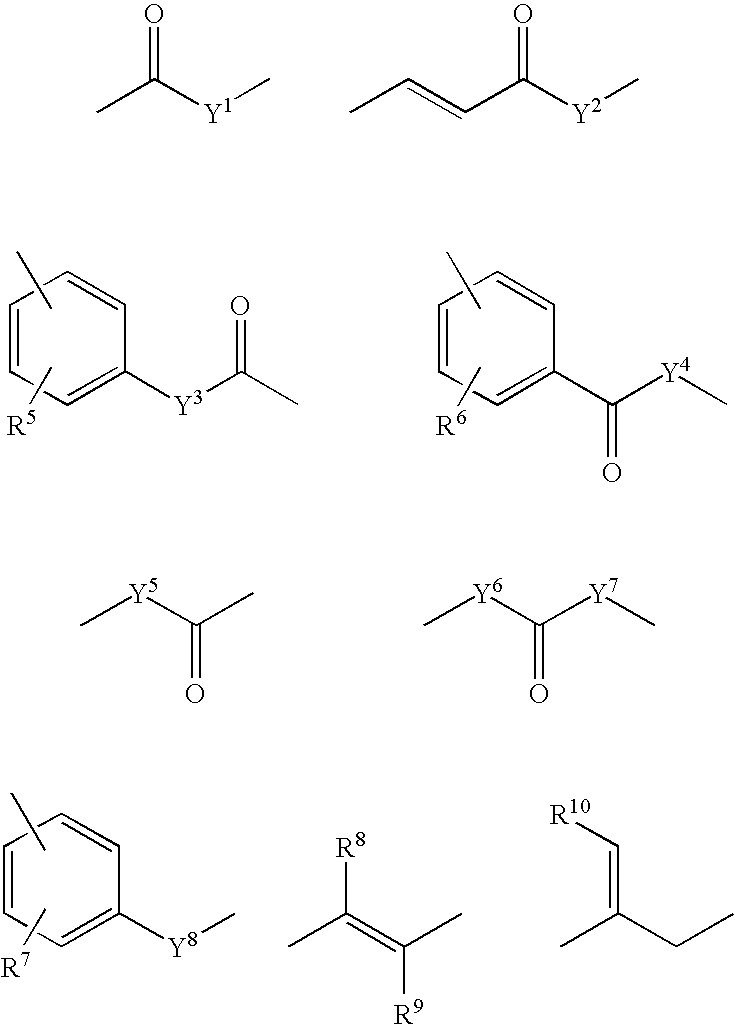
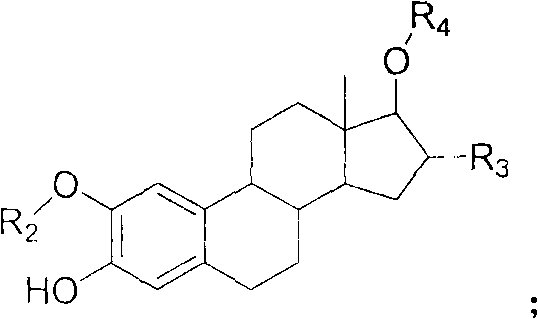
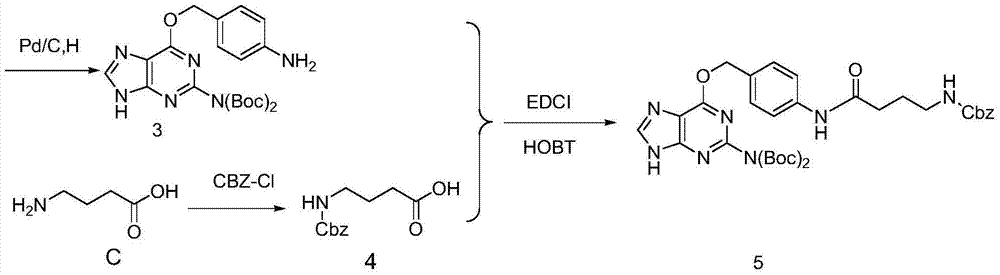
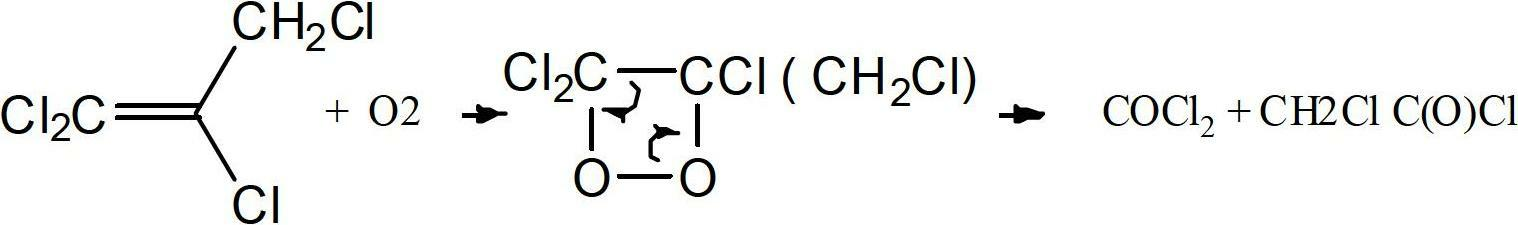
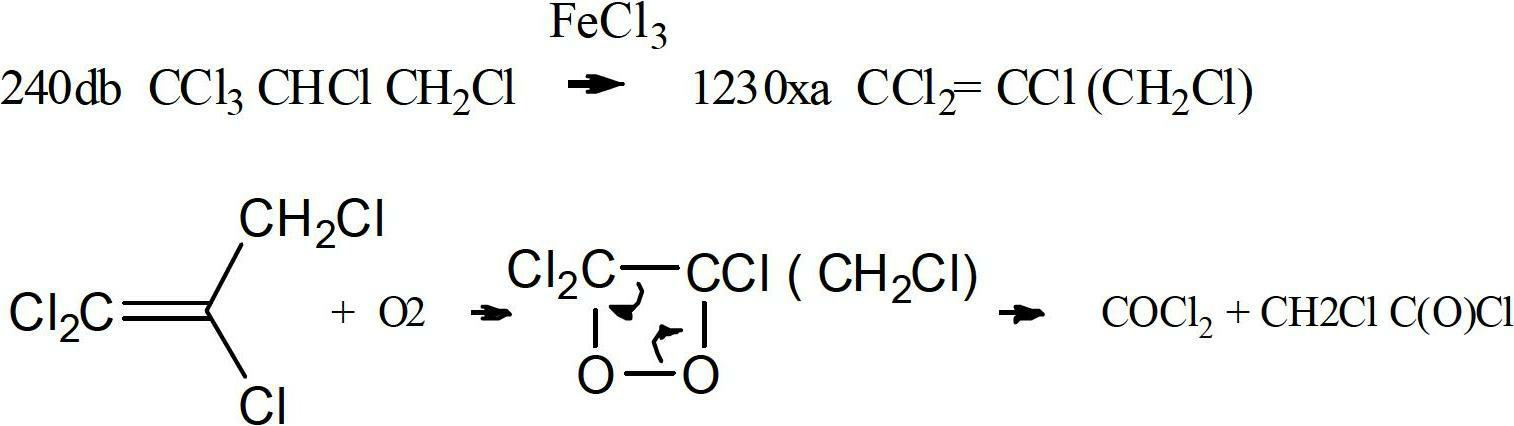
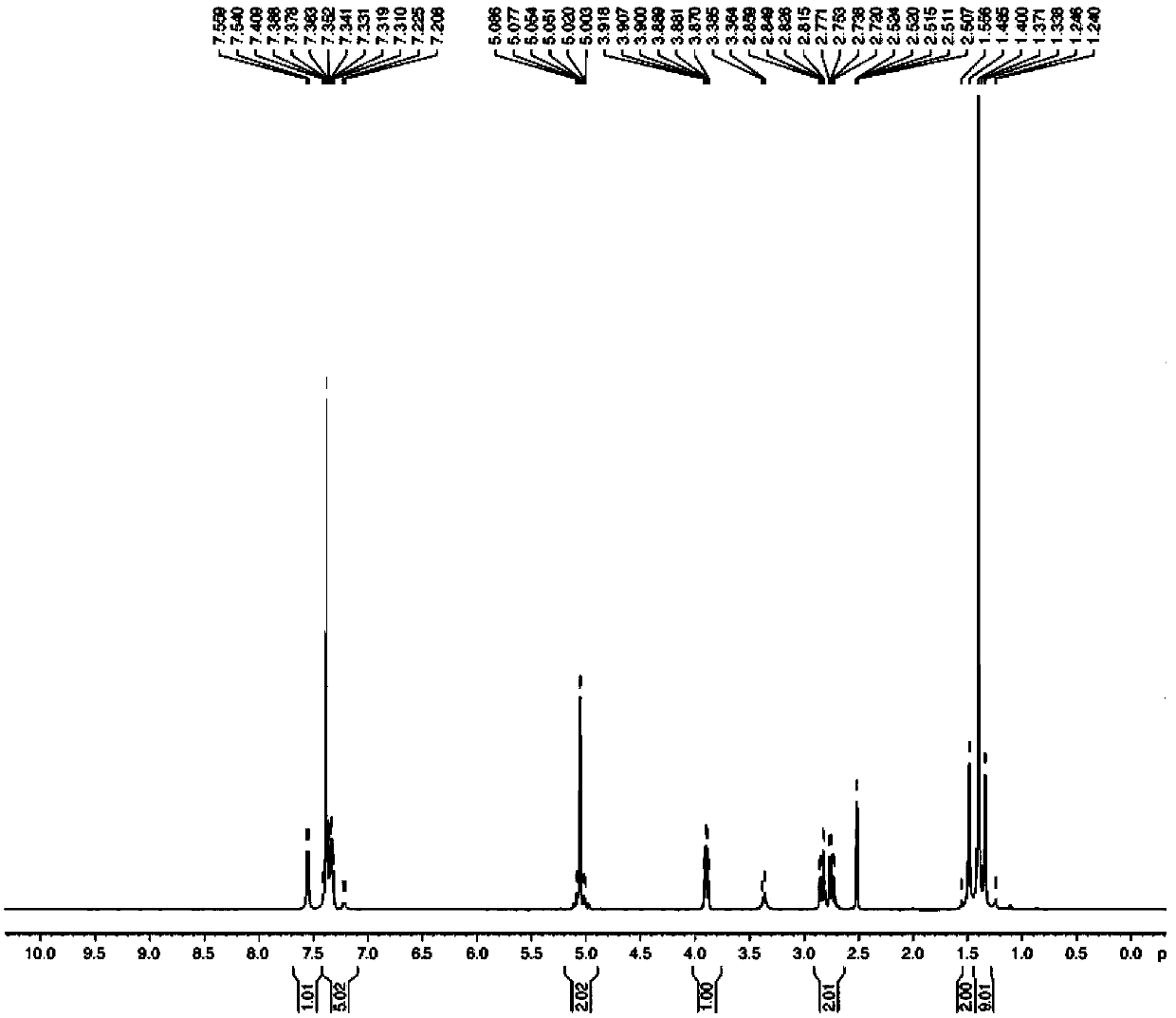
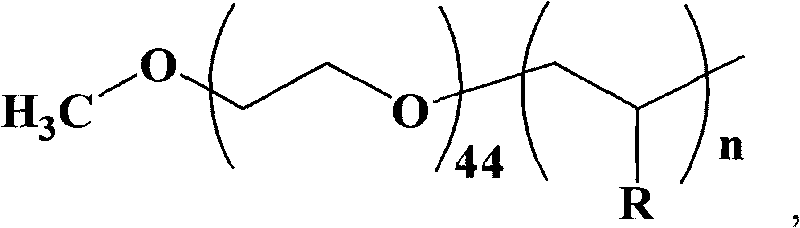
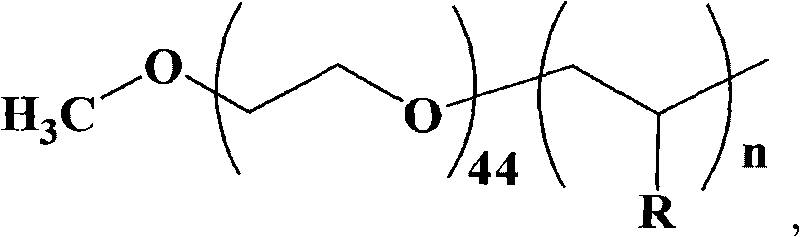
![Synthesis method of racemic-(3aR,7aR)-5-(t-butyloxycarboryl)-octahydro furan [3,2-c] pyridine-3a- carboxylic acid Synthesis method of racemic-(3aR,7aR)-5-(t-butyloxycarboryl)-octahydro furan [3,2-c] pyridine-3a- carboxylic acid](https://images-eureka.patsnap.com/patent_img/985c4940-3413-4eb4-ac6c-89b233e25011/972576DEST_PATH_IMAGE002.png)
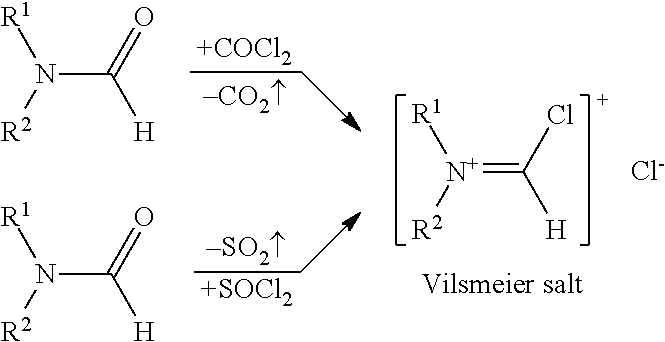
![Synthesis method of racemic-(3aR,7aR)-5-(t-butyloxycarboryl)-octahydro furan [3,2-c] pyridine-3a- carboxylic acid Synthesis method of racemic-(3aR,7aR)-5-(t-butyloxycarboryl)-octahydro furan [3,2-c] pyridine-3a- carboxylic acid](https://images-eureka.patsnap.com/patent_img/985c4940-3413-4eb4-ac6c-89b233e25011/DEST_PATH_IMAGE001.png)