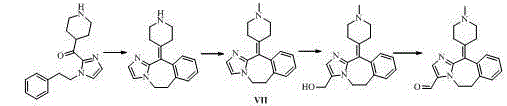
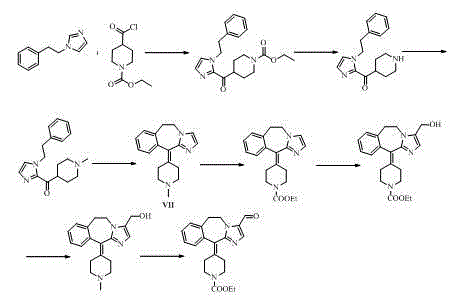
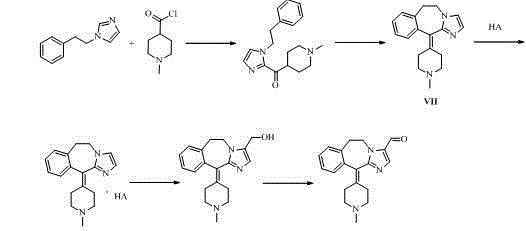
Alcaftadine intermediate and synthetic method for alcaftadine
A kind of technology of alcaftadine and synthesis method, applied in the field of synthesis of alcaftadine intermediate and alcaftadine, can solve problems such as unsuitable for industrial production, high reaction temperature, long reaction time, etc., to avoid dihydroxy Effects of methylated by-products, high synthesis yield, and short reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 5-(Trimethylsilyloxymethylene)-1 H -imidazole compound formula III Synthesis
[0048]
[0049] (1 H -Imidazol-5-yl)methanol (9.9g, 0.1mol) was added to dichloromethane (100mL), and stirred evenly. DIPEA (38.8 g, 0.3 mol) was added and stirring was continued for 30 min. Then cool to 0~5°C, add trimethylchlorosilane (TMSCl, 17.4g, 0.16mol) dropwise, stir at the same temperature for 1~2h after dropping, and concentrate the reaction solution to dryness to obtain an oil compound III .
Embodiment 2
[0051] 1-Phenylethyl-5-trimethylsilylmethylene-1 H -imidazole compound formula IV Synthesis
[0052]
[0053] The formula of the compound prepared in the previous step III and potassium carbonate (16.5g, 0.12mol) were added into acetonitrile (150mL), and stirred evenly. The temperature was raised to 75°C, and β-phenethyl bromide (18.5 g, 0.1 mol) was slowly added dropwise. After the dropwise addition, the reaction was continued for 5h. Cool to room temperature, filter with suction, concentrate the filtrate to dryness, dissolve the remaining oil in a mixed solvent of dichloromethane and water, add concentrated hydrochloric acid dropwise to pH=3~4, separate the liquids after standing still, take the water layer, and add sodium bicarbonate Solid, to pH=7~8, and no bubbles, dichloromethane extracted 3 times, the organic phase was concentrated to dryness, and the oil was purified by column to obtain the compound formula IV (14.5g, 85 % ). 1 H-NMR (300 MHz, DMSO-d 6 , ...
Embodiment 3
[0055] (5-(Hydroxymethyl)-1-phenyl-1 H -imidazol-2-yl)(1-methylpiperidin-4-yl)methanol compound formula V Synthesis
[0056]
[0057] Compound formula IV (13.7g, 0.05mol) and triethylamine (12.3g, 0.12mol) were added into acetonitrile (200mL), and stirred evenly. Cool to 0-5°C, and slowly add 1-methylpiperidine-4-carbonyl chloride hydrochloride (13.8 g, 0.07 mol) newly prepared in advance in batches. After the addition was complete, it was transferred to room temperature for 20 h. Add dropwise 40% aqueous sodium hydroxide solution to pH=9~10, then concentrate to dryness. Dissolve the remaining solid in water and ethyl acetate, add concentrated hydrochloric acid dropwise to pH = 3~4, separate the liquid after standing still, take the water layer, add solid sodium bicarbonate until pH = 7~8, and no bubbles are generated. Stir and crystallize at 5°C for 4~5h, filter with suction, and wash the filter cake with water. The obtained filter cake is dried to obtain the compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com