Aromatic helical foldamer as well as preparation method and application of aromatic helical foldamer
A folded body and helix technology, applied in the field of aromatic helical folded body and its preparation, can solve the problems of formation mechanism and specific conformational arrangement research difficulties, and achieve the effect of great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
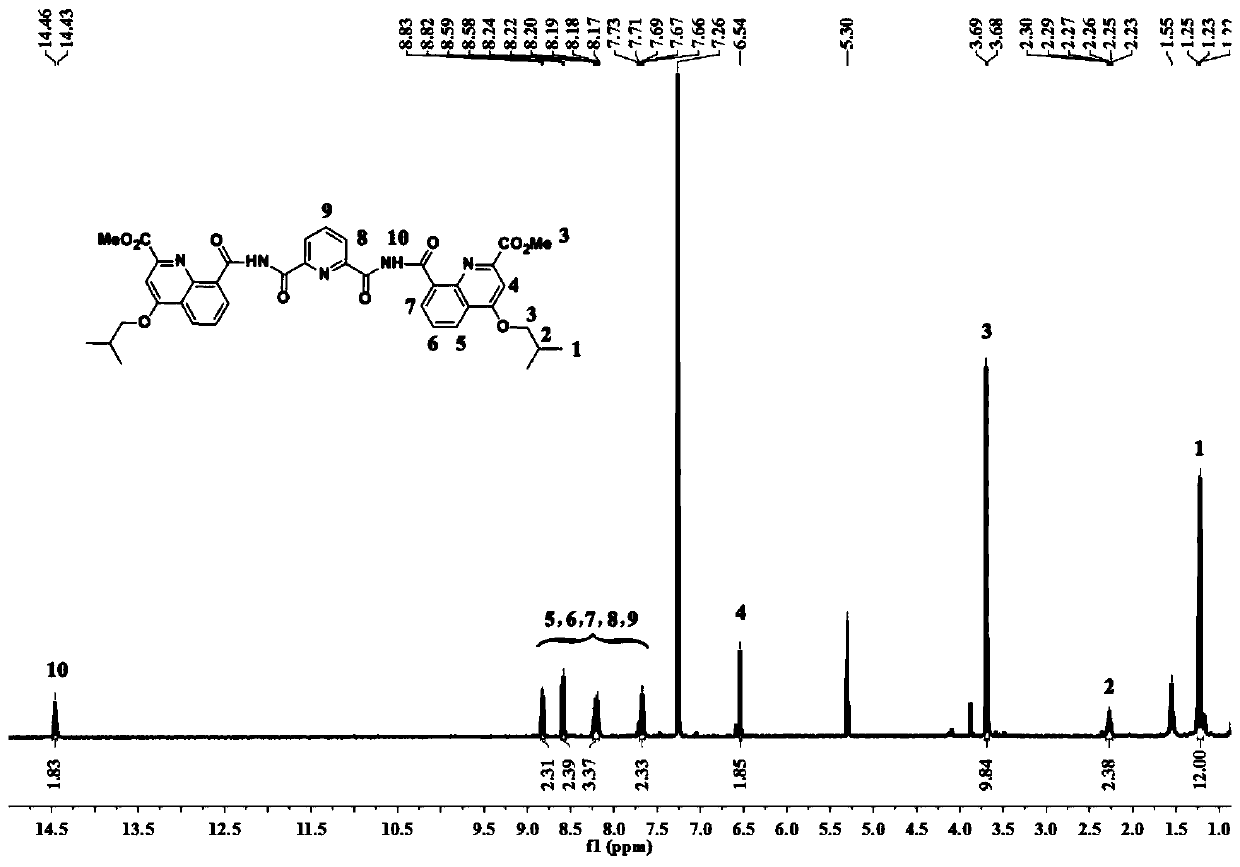
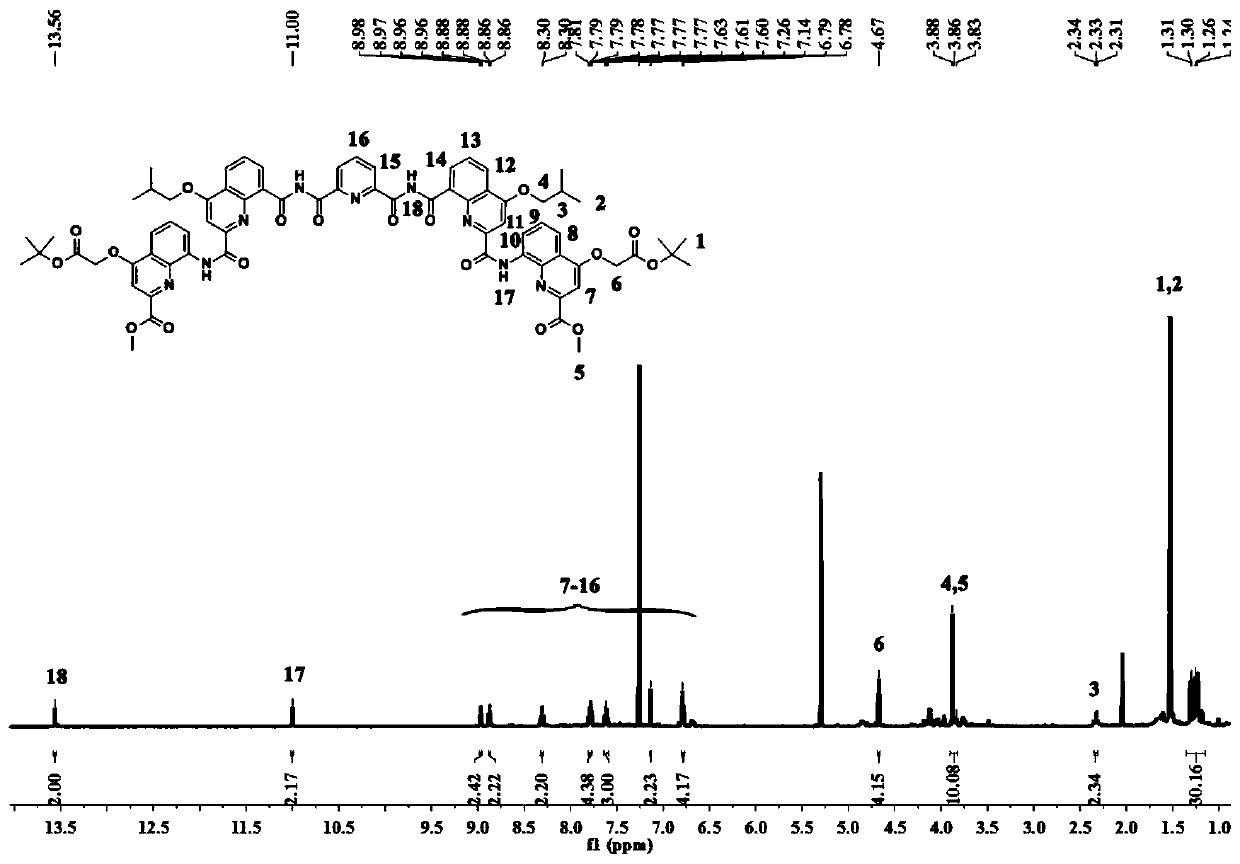
[0051] The synthetic route of preparing the trimer oligomer containing bisacyl chloride active group is as follows:
[0052]
[0053]
[0054] The preparation method specific steps of the trimer oligomer containing bisacyl chloride active group are as follows:
[0055] Step A: Dissolve 10g of 2-aminobenzamide in a round-bottomed flask with 30ml of anhydrous methanol, feed 10g of 2-aminobenzamide and dimethyl butyndioate in a molar ratio of 1:1.1, and Dissolve dimethyl alkynedioate in 15ml of anhydrous methanol, then slowly add it dropwise into a round bottom flask, stir at room temperature until it is evenly mixed, and heat to reflux for 8 hours.
[0056] Let stand to room temperature, and put in a -20°C refrigerator overnight. The next day, filter and wash the filter cake repeatedly with ice methanol until the filtrate is colorless and clear. The bright yellow product 1,2-(2-carbamoylphenyl)amino)maleic acid dimethyl was collected after being fully dried at 30° C. in ...
Embodiment 2
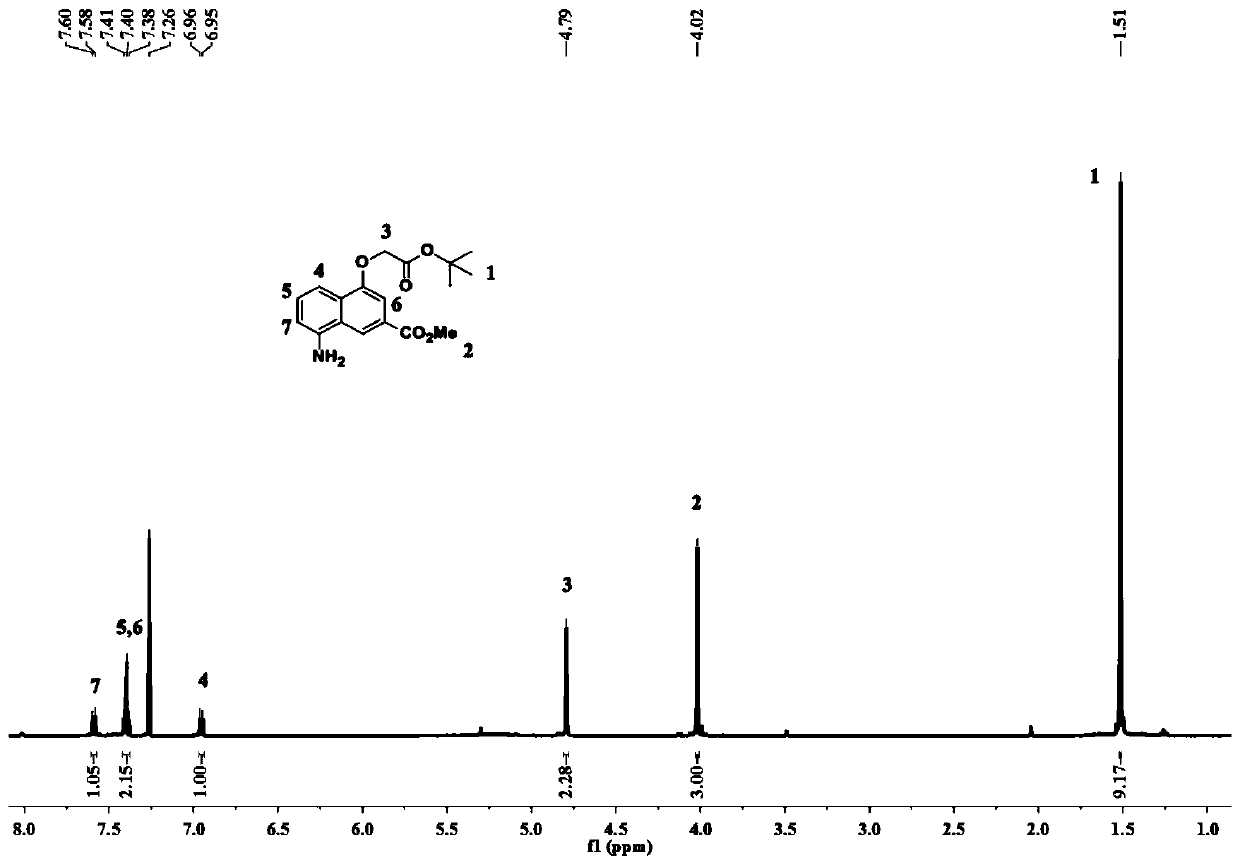
[0087] The synthetic route of preparing the trimer oligomer containing bisacyl chloride active group is as follows:
[0088]
[0089]
[0090] The specific steps of the preparation method of the trimer containing the bisacyl chloride active group are as follows:
[0091] Step A: Dissolve 10g of 2-aminobenzamide in a round-bottomed flask with 30ml of anhydrous methanol, feed 10g of 2-aminobenzamide and dimethyl butynedate at a molar ratio of 1:1.3, and first add Dissolve dimethyl alkynedioate in 17ml of anhydrous methanol, then slowly add it dropwise into a round bottom flask, stir at room temperature until it is evenly mixed, and heat to reflux for 8 hours.
[0092] Allow to cool to room temperature and place in a -20°C refrigerator overnight. The next day, suction filtration was performed and the filter cake was repeatedly washed with ice methanol until the filtrate was colorless and clear. Finally, the filter cake was put into a vacuum drying oven, and after being fu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com