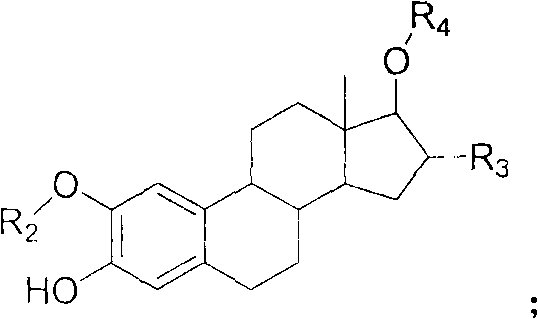
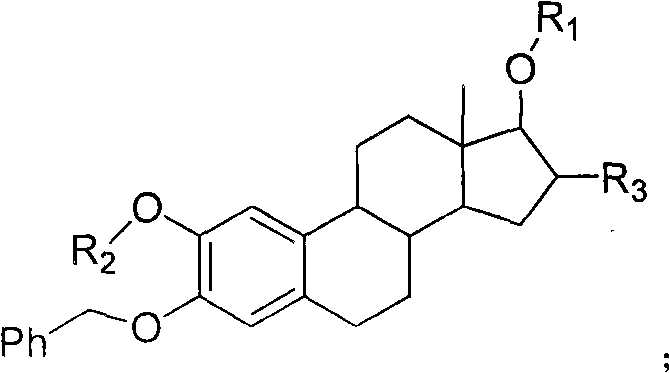
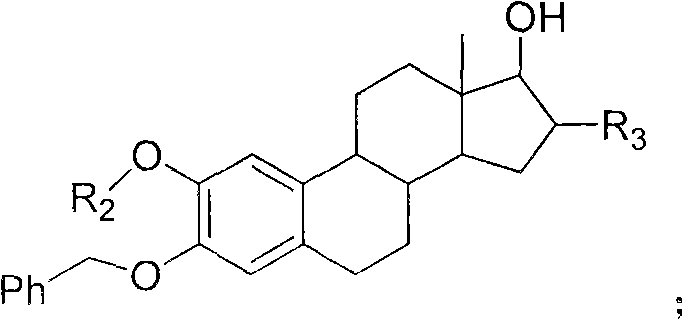
Estrogen amino-acid ester compound with antitumor activity as well as synthetic method thereof
A technology of steroidal amino acid ester and anti-tumor activity, applied in the field of medicine, can solve the problems of poor water solubility, limited wide application, low bioavailability, etc., and achieve the effect of good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of 2-methoxy-3,17β-dihydroxy-1,3,5(10)-estratriene-17-β-alanine ester
[0035] (1), the preparation of N-benzyloxycarbonyl-beta-alanine
[0036] At 5°C, 10 mmol of β-alanine was dissolved in 4.0 ml of 10% sodium hydroxide (NaOH) aqueous solution to obtain a solution, and 14 mmol (i.e. 1.8 ml) of benzyl chloroformate was added while stirring. Add dropwise to the solution solution at a speed of 1 drop / 4 seconds for reaction, and add dropwise a 10% sodium hydroxide (NaOH) aqueous solution to keep the pH at 8. After the drop is complete, place it at 10°C and stir for 15 minutes. After 1 hour, use ninhydrin as the color developing agent to develop the color without purple color, then wash with 5ml / time ether for 3 times, discard the ether to obtain the aqueous phase, and adjust the pH of the aqueous phase to 3 with concentrated hydrochloric acid with a mass concentration of 30%. A milky white solid appeared, and the liquid after the milky white solid ...
Embodiment 2
[0041] Example 2: Preparation of 16α-ethyl-2-methoxy-3,17β-dihydroxy-1,3,5(10)-estratriene-17-L-phenylalanine ester
[0042] (1), the preparation of N-benzyloxycarbonyl-L-phenylalanine
[0043] At -3°C, 10 mmol of L-phenylalanine was dissolved in 4.0 ml of 9% sodium hydroxide (NaOH) aqueous solution to obtain a solution, and 14 mmol (i.e. 1.8 ml) of chloroformic acid was added while stirring. The benzyl ester is added dropwise to the solution at a speed of 1 drop / 5 seconds for reaction, and a 9% sodium hydroxide (NaOH) aqueous solution is added dropwise to keep the pH at 8. After the drop is completed, place it at 15°C and stir React for 15 hours, do TLC detection, after the solution is completely reacted, wash 3 times with 5ml / time of diethyl ether, discard the diethyl ether to obtain the water phase, and adjust the pH of the water phase to 2 with concentrated hydrochloric acid with a mass concentration of 35%. , a milky white solid appeared, and the liquid after the milky w...
Embodiment 3
[0048] Example 3: 16α-(N,N-dimethyl)aminomethyl-2-methoxy-3,17β-dihydroxy-1,3,5(10)-estratriene-17-L-leu Preparation of Amino Acid Esters
[0049] (1), the preparation of N-benzyloxycarbonyl-L-leucine
[0050] At 2°C, 10 mmol of L-leucine was dissolved in 4.0 ml of 8% sodium hydroxide (NaOH) aqueous solution to obtain a solution, and 14 mmol (i.e. 1.8 ml) of benzyl chloroformate was added while stirring. Add dropwise to the solution solution at a speed of 1 drop / 6 seconds for reaction, and add dropwise an aqueous solution of sodium hydroxide (NaOH) with a mass concentration of 8% to keep the pH at 9. After the drop is complete, place it at 20°C and stir for 15 minutes. After 1 hour, use ninhydrin as the color developing agent to develop the color without purple color, then wash with 5ml / time of diethyl ether for 3 times, discard the diethyl ether to obtain the aqueous phase, and adjust the pH of the aqueous phase to 1 with concentrated hydrochloric acid with a mass concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com