Preparation method of mesosulfuron-methyl
The technology of methyldisulfuron-methyl and methoxycarbonylbenzenesulfonamide is applied in the field of preparation of herbicides, can solve the problems of removing impurities, many impurities and the like, and achieves the effects of high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0028] The preparation method of methyldisulfuron-methyl of the present embodiment comprises the following steps:
[0029] ① Preparation of isocyanate.
[0030] Add 700g of dichloromethane and 35g of bis(trichloromethyl)carbonate to a dry 1000mL reaction flask, stir, and after the bis(trichloromethyl)carbonate is dissolved, cool the solution to 0-5 ℃, and add it to bis(trichloromethyl)carbonate. Trichloromethyl) carbonate solution was added dropwise with pyrimidinamine (compound III, 2-amino-4,6-dimethoxypyrimidine) solution (0.226 mol, 35 g of pyrimidinamine was dissolved in 100 g of dichloromethane), added After stirring for 20-60 min (30 min in this example), 100 g of acid binding agent triethylamine was added dropwise, and after the addition was completed, the mixture was stirred for 0.5-1 h to obtain an isocyanate (compound IV) solution for later use. During the reaction, the temperature was controlled below 50°C.
[0031] In addition to the above-mentioned dichlorometh...
Embodiment 2)
[0039] The rest of the preparation method of the methyldisulfuron-methyl of the present embodiment is the same as in Example 1, and the difference is:
[0040] Step 1 When preparing isocyanate, add 700 g of dichloromethane and 35 g of trichloromethyl chloroformate into a dry 1000 mL reaction flask.
[0041] The above-mentioned diphosgene and triphosgene can also be replaced by phosgene.
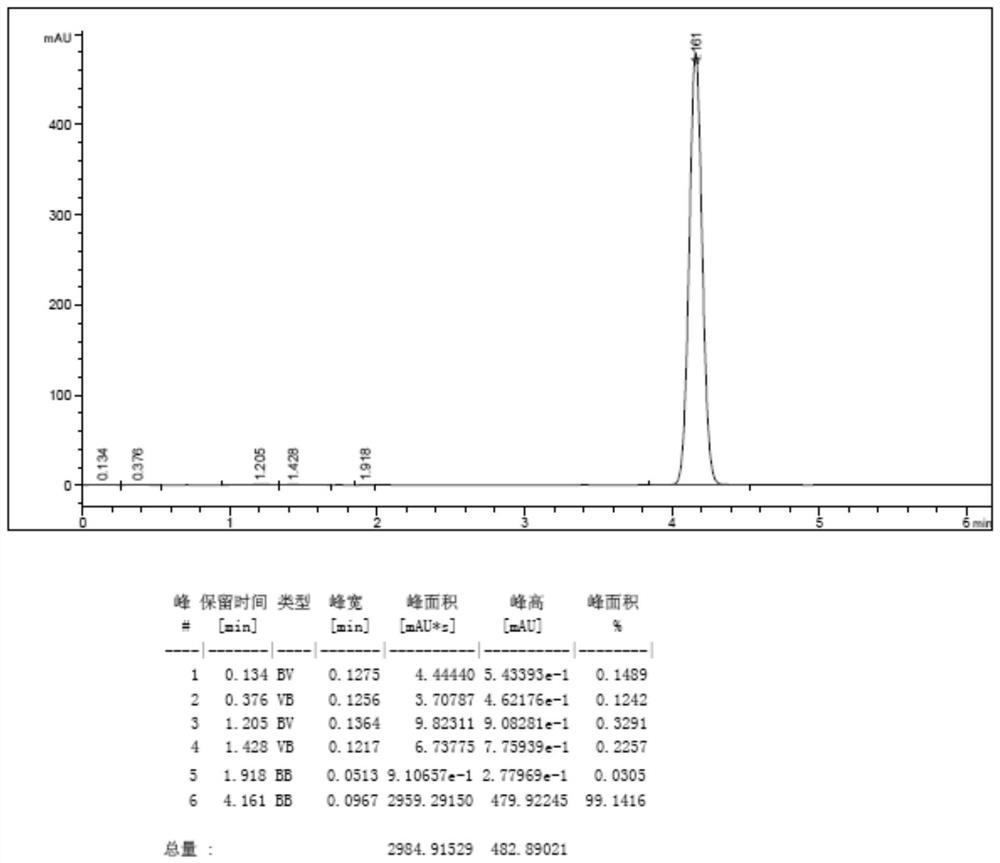
[0042] Step 2. After drying, 100 g of milky white powdery solid was obtained, the yield was 92%, and the purity detected by HPLC was 98.6% (see the HPLC detection map). figure 2 ).
Embodiment 3)
[0044] The rest of the preparation method of the methyldisulfuron-methyl of the present embodiment is the same as in Example 1, and the difference is:
[0045] Step 1 When preparing isocyanate, add 700g of toluene and 35g of bis(trichloromethyl)carbonate to a dry 1000mL reaction flask, stir, and cool the solution to 0~5 ℃ after the bis(trichloromethyl)carbonate is dissolved , add dropwise pyrimidineamine solution (0.226 mol, disperse 35g pyrimidineamine in 100g toluene to obtain suspension) to bis(trichloromethyl)carbonate solution, stir for 30min after adding, and then add dropwise 100g acid binding agent three Ethylamine was added and stirred for 1 h to obtain an isocyanate solution for later use.
[0046] The solvent in step ② is toluene.
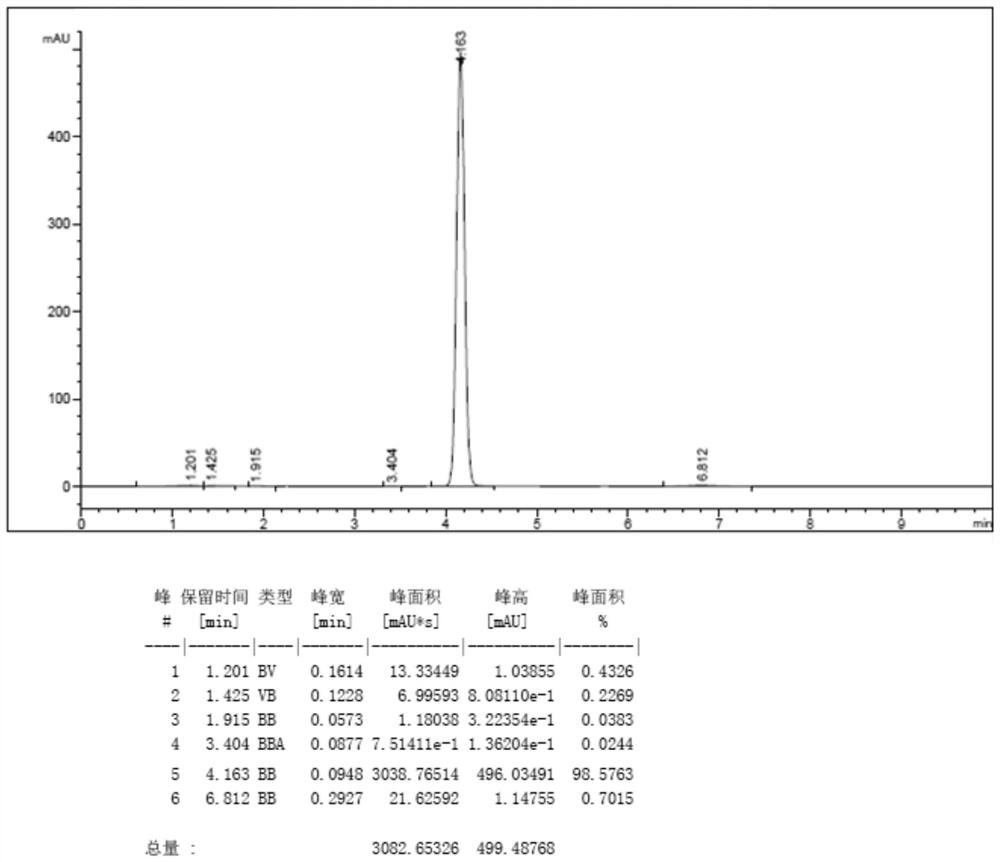
[0047] Step 2. After drying, 102 g of milky white powdery solid was obtained, the yield was 93%, and the purity detected by HPLC was 98.2% (see the HPLC detection map). image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com