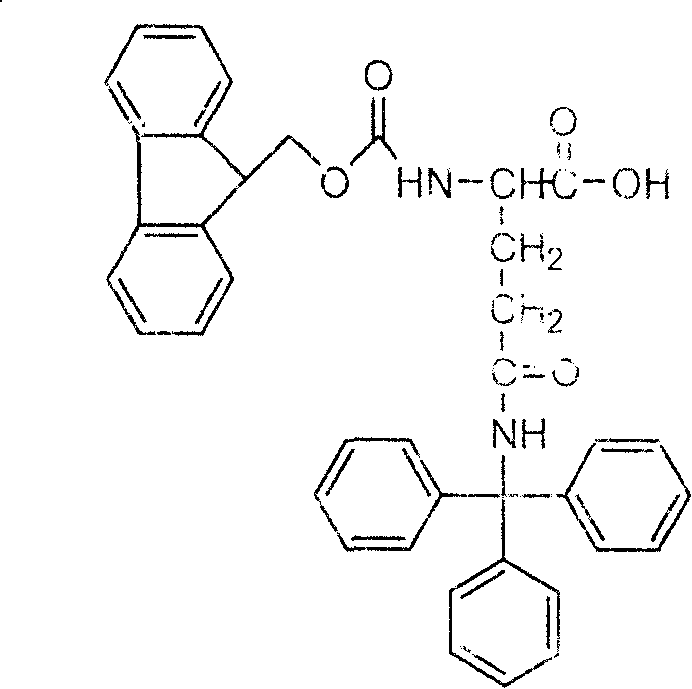
Method for synthesizing N-fluorene methoxycarbonyl-N-trityl-D-glutamine
A technology of fluorene methoxycarbonyl and trityl, which is applied in the synthesis field of N-fluorene methoxycarbonyl-N-trityl-D-glutamine-OH), and can solve the problem of D-glutamine synthesis The problem of high cost, to achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
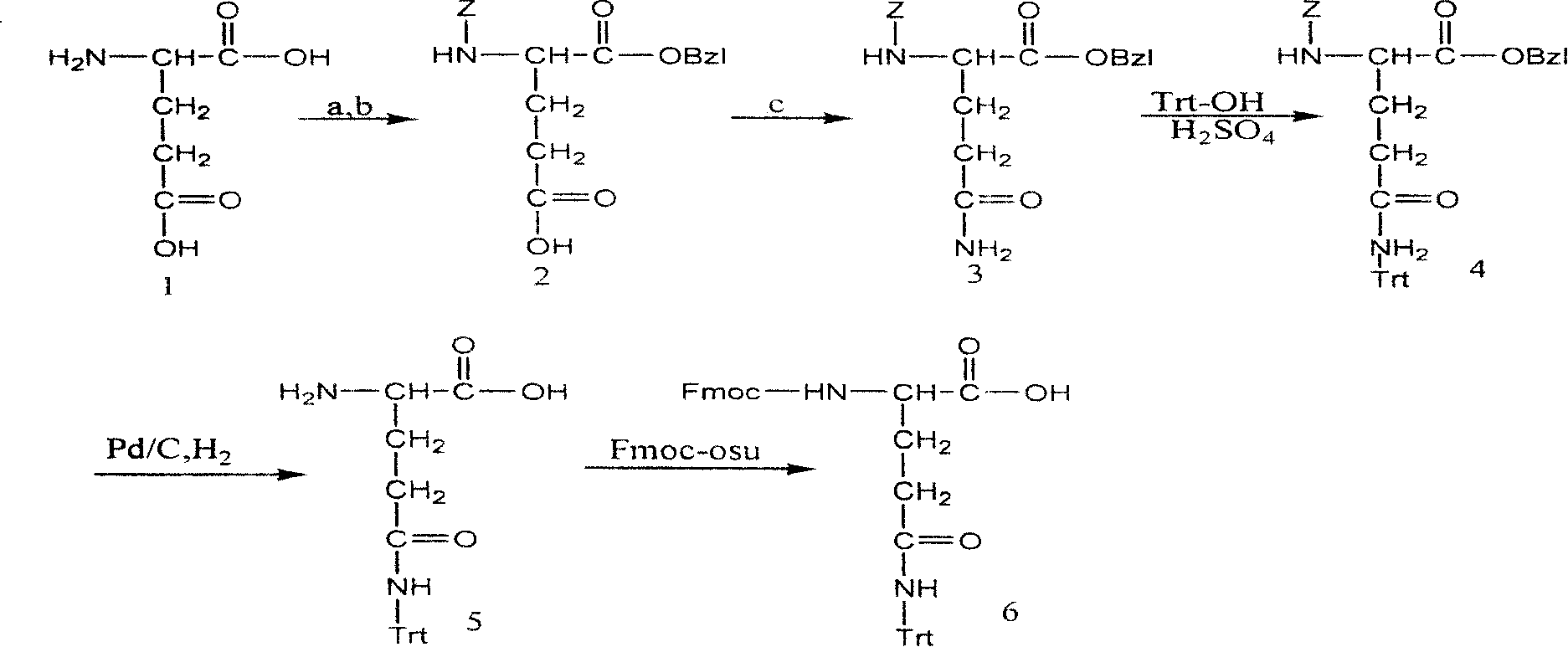
[0036] Example 1, referring to the synthetic route, react glutamic acid and Z-Cl in sodium hydroxide solution at 0-15°C to obtain Z-D-Glu-OH. Z-D-Glu-OH was dissolved in DMF, and triethylamine and benzyl bromide were added to react for 24 hours to obtain Z-D-Glu-OBzl. Dissolve 10mmol Z-D-Glu-OBzl in 100mlTHF, add 10mmolET 3 N, add 10mmol of ClCOOEt at -10~-20°C, add 60mmol of ammonia water after 15-30min, react at room temperature for 6hr, and post-process to obtain product 3 with a yield of 62%. Dissolve 10 mmol of compound 3 and 20 mmol of trityl alcohol in 300 ml of HAc, add 1 mmol of sulfuric acid and 20 mmol of acetic anhydride, react at 60° C. for 8 hours, and post-process to obtain product 4 with a yield of 50%. 10 mmol of compound 4 was dissolved in methanol, 0.3 g of 10% Pd / C was added, and reacted with hydrogen gas for 24 hrs to obtain product 5 with a yield of 92%. Dissolve 10 mmol of compound 5 in saturated sodium bicarbonate and dioxane, add 10 mmol of Fmoc-Osu,...
Embodiment 2
[0037] Example 2, dissolve 10mmol Z-D-Glu-OBzl in 100mlTHF, add 10mmolET 3 N, add 10mmol ClCOOEt at -10~-20°C, add 40mmol ammonia water after 15-30min, react at room temperature for 16hr, and post-process to obtain product 3 with a yield of 60.5%. Dissolve 10 mmol of compound 3 and 20 mmol of trityl alcohol in 300 ml of HAc, add 1 mmol of sulfuric acid and 20 mmol of acetic anhydride, react at 50° C. for 24 hours, and post-process to obtain product 4 with a yield of 48%. 10 mmol of compound 4 was dissolved in methanol, 0.6 g of 5% Pd / C was added, and reacted with hydrogen gas for 36 hrs to obtain product 5 with a yield of 91.5%. All the other are identical with embodiment 1.
Embodiment 3
[0038] Example 3, dissolve 10mmol Z-D-Glu-OBzl in 100ml ethyl acetate, add 10mmolET 3 N, add 10mmol ClCOOEt at -10~-20°C, add 20mmol ammonia water after 15-30min, react at room temperature for 24hr, and post-process to obtain product 3 with a yield of 60%. Dissolve 10 mmol of compound 3 and 20 mmol of trityl alcohol in 300 ml of HAc, add 1 mmol of sulfuric acid and 20 mmol of acetic anhydride, react at 45°C for 24 hrs, and post-process to obtain product 4 with a yield of 47%. 10 mmol of compound 4 was dissolved in methanol, 0.6 g of 10% Pd / C was added, 10 ml of cyclohexene was added and refluxed for 8 hours, and post-treatment was performed to obtain product 5 with a yield of 90%. Dissolve 10 mmol of compound 5 in saturated sodium bicarbonate and dioxane, add 10 mmol of Fmoc-Cl, react for 4 hours, and post-process to obtain product 6. All the other are identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com