Preparation method and applications of oriented immobilized PEGA composite resin
A composite resin technology, applied in the field of biomedicine, can solve the problems of active site influence, protein loss, weak interaction between protein and solid materials, etc., and achieve the effect of simple reaction steps and stable reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
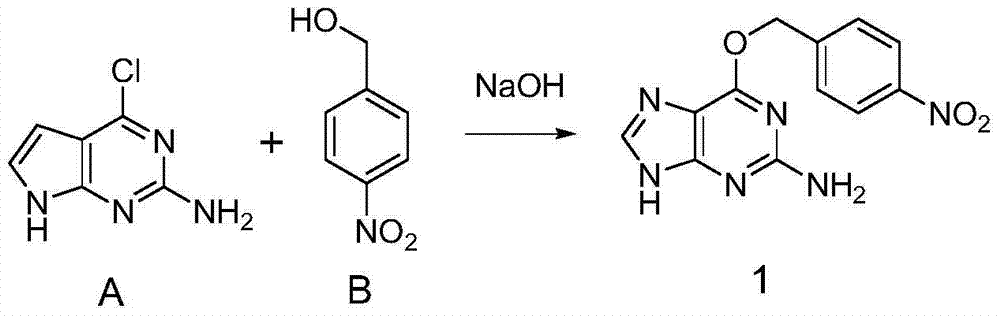
[0025] a. Add 2-amino-6-chloropurine (compound A) and 4-nitrobenzyl alcohol (compound B) into methanol, the reaction temperature is 80°C, the molar ratio of the reaction substrate is 1:1, and the heating and reflux time is 2 The Williamson ether formation reaction occurred under alkaline conditions for 4 hours to generate 4-nitro-benzylguanine; the yield was 95%. The following reaction formula is shown:
[0026]
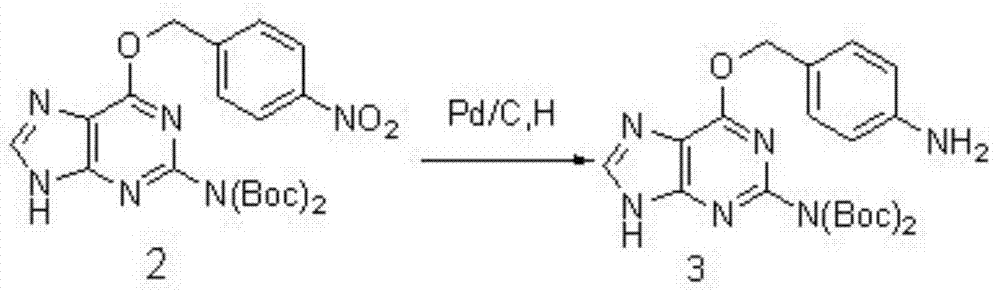
[0027] b, 4-nitro-benzylguanine is protected by tert-butoxycarbonyl group, hydrogenation under normal pressure under the catalysis of palladium carbon consumption being 5% of the reaction product, dehydrated tetrahydrofuran is added dropwise, in 4-dimethylaminopyridine Under the condition of existence, di-tert-butyl dicarbonate was added, the reaction temperature was 25° C., and the stirring time was 6 hours, and the compound (3) was reduced to generate compound (3); the yield was 97%. The following reaction formula is shown:
[0028]
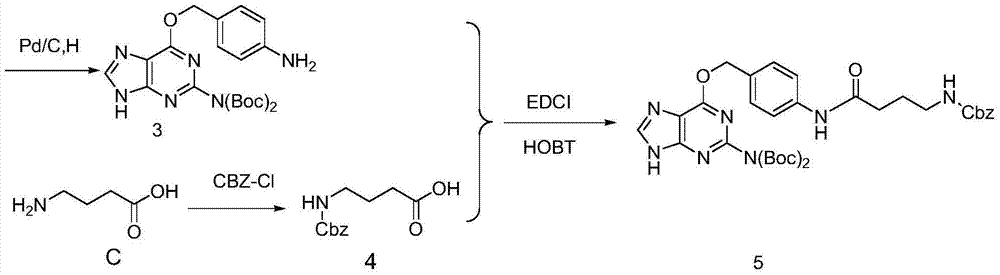
[0029] c. Compound (3)...
Embodiment 2
[0037] a. Add 2-amino-6-chloropurine (compound A) and 4-nitrobenzyl alcohol (compound B) into ethanol, the reaction temperature is 80°C, the molar ratio of the reaction substrate is 1:1, and the heating time is 6 The Williamson ether formation reaction occurred under alkaline conditions for 4 hours to generate 4-nitro-benzylguanine; the yield was 96%. The following reaction formula is shown:
[0038]
[0039] b, 4-nitro-benzylguanine is protected by tert-butoxycarbonyl group, hydrogenation under normal pressure under the catalysis of palladium carbon consumption being 5% of the reaction product, dehydrated tetrahydrofuran is added dropwise, in 4-dimethylaminopyridine Under the condition of existence, di-tert-butyl dicarbonate was added, the reaction temperature was 25° C., and the stirring time was 7 hours, and the compound (3) was reduced; the yield was 98%. The following reaction formula is shown:
[0040]
[0041] c. Compound (3), the γ-aminobutyric acid protected b...
Embodiment 3
[0048] a. Add 2-amino-6-chloropurine (compound A) and 4-nitrobenzyl alcohol (compound B) into isopropanol, the reaction temperature is 80°C, the molar ratio of the reaction substrate is 1:1, and the heating time is reflux The Williamson ether formation reaction occurred under alkaline conditions for 12 hours to generate 4-nitro-benzylguanine; the yield was 98%. The following reaction formula is shown:
[0049]
[0050] b, 4-nitro-benzylguanine is protected by tert-butoxycarbonyl group, hydrogenation under normal pressure under the catalysis of palladium carbon consumption being 5% of the reaction product, dehydrated tetrahydrofuran is added dropwise, in 4-dimethylaminopyridine Under the condition of existence, di-tert-butyl dicarbonate was added, the reaction temperature was 25° C., and the stirring time was 8 hours, and the compound (3) was reduced; the yield was 99%. The following reaction formula is shown:
[0051]
[0052] c. Compound (3), the γ-aminobutyric acid p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com