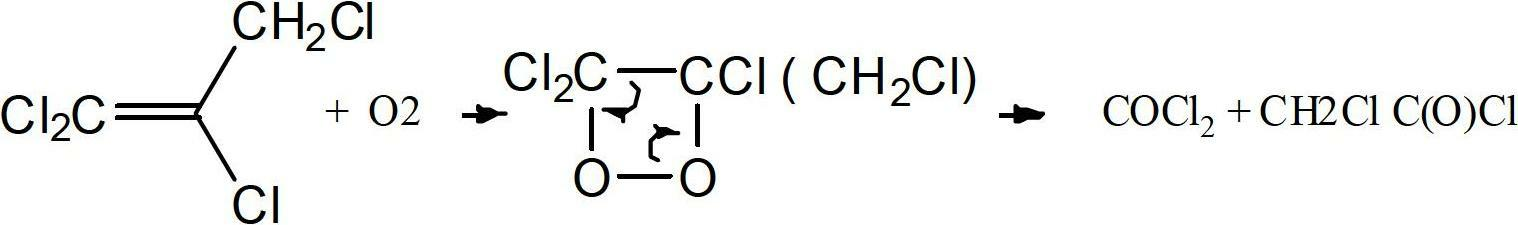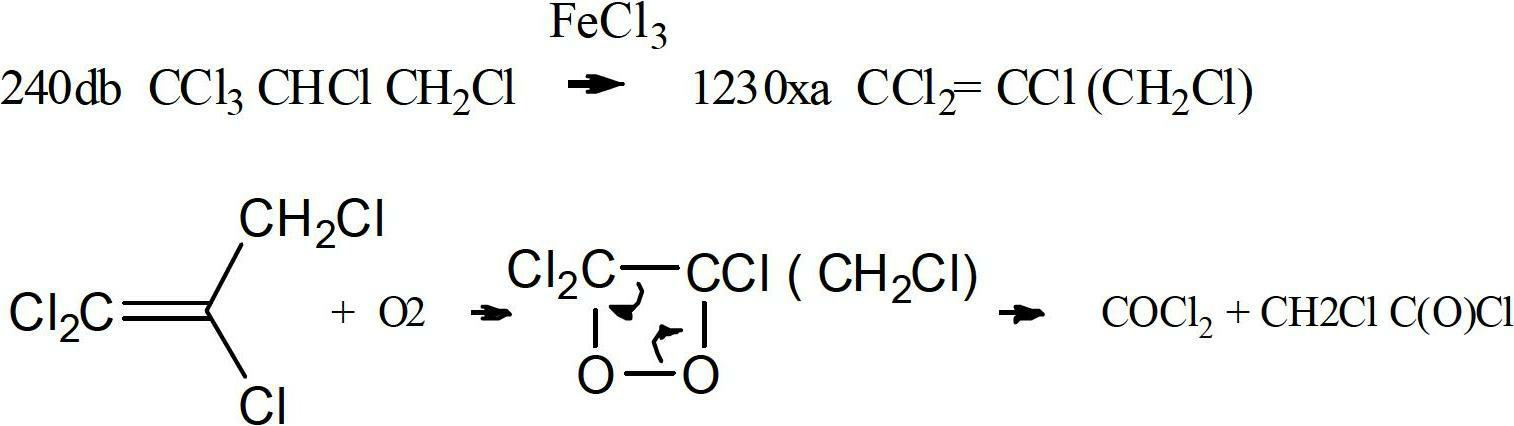Method to purify and stabilize chloroolefins
A technology of chloroalkenes and tetrachloropropene, which is applied in the field of stable compositions of chloroalkenes, and can solve problems such as the difficulty of processing on a commercial scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] Example 1 Batch purification of 1230xa using activated alumina
[0026] Chloroolefin 1230xa (120 g, 14 ppm phosgene) can be mixed with 50 g of various commercially available dried activated aluminas such as La Roche A201, A204 and BASF AL-4126E / 16, and The analysis was performed after approximately one and a half hours at room temperature. Analysis of phosgene can be performed spectrophotometrically using 4-(p-nitrobenzyl)-pyridine. It is often expected to show that the level of phosgene is reduced to less than about 1 ppm.
example 2
[0027] Example 2 Continuous uptake of phosgene from 1230xa using alumina
[0028] Chloroolefin 1230xa, containing about 14 ppm phosgene, can be fed through a fixed bed of about 85 grams of LaRoche 204 or BASF AL-4126E / 16 at room temperature at a rate of 20 ml / min. The contact time will be about 5.3 minutes. It is often expected that the phosgene level per alumina tested is less than about 4 ppm.
example 3
[0029] The effect of example 3 contact time on phosgene absorption
[0030]Example 2 using La Roche 204 alumina can be repeated using about 8 grams of activated alumina and a contact time of about 53 minutes. The phosgene level is often expected to be reduced to about 4 ppm. At a contact time of about 26 minutes, the phosgene level is often expected to be reduced to about 1 ppm.
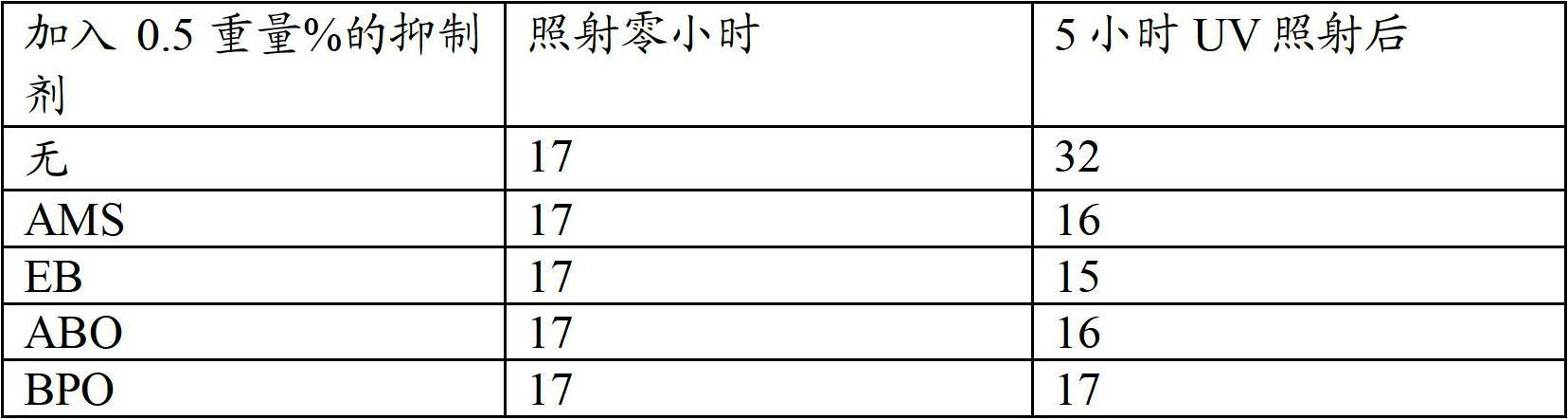
[0031] The test of example 41230xa chemical stability
[0032] Alumina-treated 1230xa from Example 2 (containing about 5% by weight α-methylstyrene) was subjected to UV irradiation in the presence of air for about five hours, simulating about one year of aging at ambient temperature. It is often expected that the analysis will show no formation of phosgene. If untreated 1230xa was irradiated under similar conditions than before, formation of about 20 ppm phosgene would often be expected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com