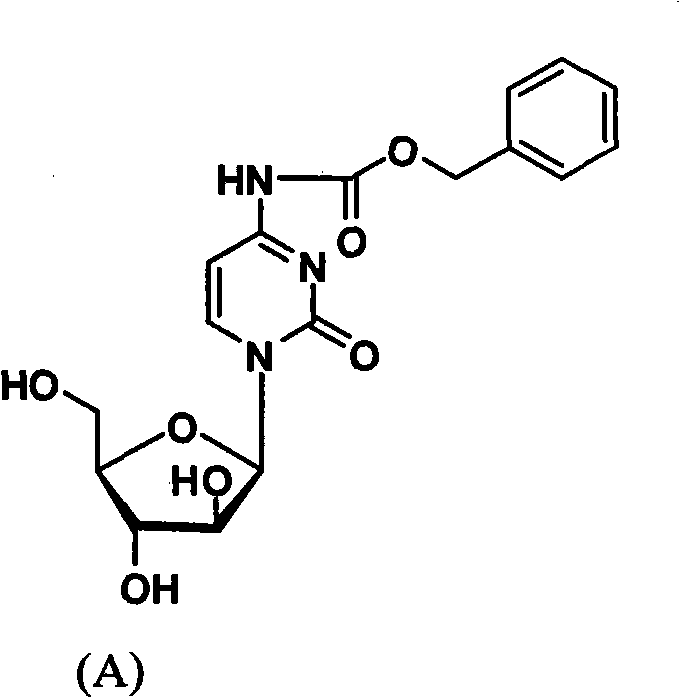
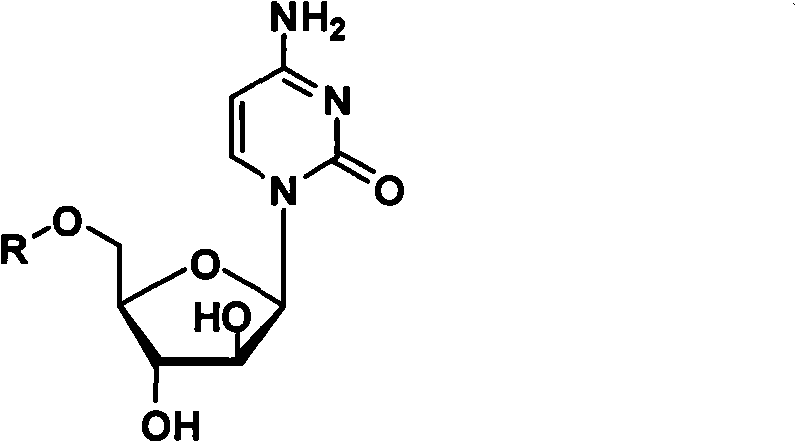
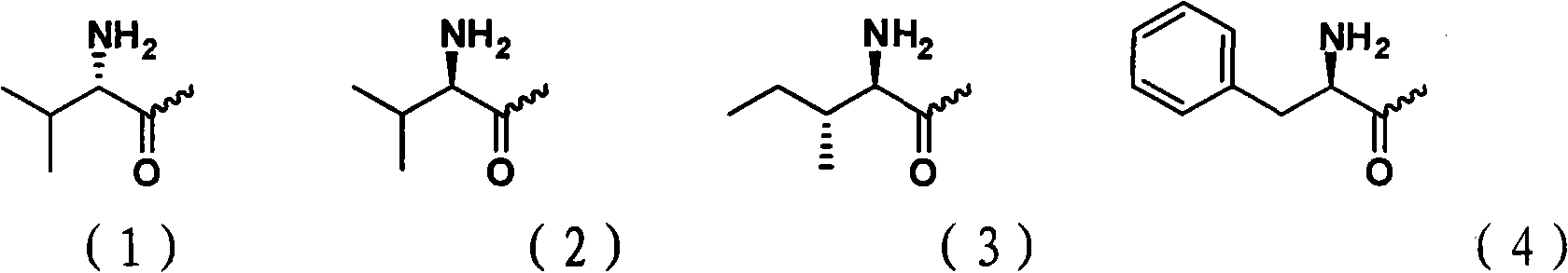
Cytarabine 5'-O-amino-acid ester, salts thereof and preparation method thereof
A cytarabine and amino acid ester technology, which is applied in the field of medicine, can solve the problems of poor small intestinal membrane permeability, poor oral bioavailability of cytarabine, and poor cytarabine membrane permeability, etc., so as to improve the membrane permeability. The effect of permeability
Inactive Publication Date: 2010-08-25
SHENYANG PHARMA UNIVERSITY
View PDF3 Cites 20 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the polarity of the cytarabine molecule is very large, resulting in poor membrane permeability of the small intestine, so the poor membrane permeability makes the oral bioavailability of cytarabine very low (about 20%)
Therefore, it is necessary to find a way to improve the poor membrane permeability of cytarabine, and then improve its oral bioavailability
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention belongs to the technical field of medicines and discloses cytarabine 5'-O-amino-acid ester, pharmaceutically acceptable salts thereof and a preparation method thereof. The preparation method comprises the following steps of: slowly dropping carbobenzoxy chloride into a solution formed by cytarabine, sodium bicarbonate and N,N-dimethylacetylamide, and obtaining a compound A after reacting at room temperature; using the compound A and N-butyloxy formoxyl-amino acid as raw materials; adding a reagent to the solution to carry out an esterification reaction to obtain the cytarabine 5'-O-amino-acid ester; and then adding acid to obtain a finished product. The pharmaceutically acceptable salts comprise hydrochlorides, sulfates, formates, acetates, mesylates, propionates, butyrates, p-toluene sulphonates, phosphates, bisulfates, maleates, lactates, carbonates, bicarbonates, malonates, and salts formed with acidic amino acids, and the like. The invention can obviously improve the membrane permeability of the cytarabine so as to improve the bioavailability of the cytarabine.
Description
Technical field: The invention belongs to the technical field of medicine, and relates to cytarabine 5'-O-amino acid esters and salts thereof and preparation methods thereof, in particular to a drug 1-β-D-arabinofuranosyl-4-amino- 5'-O-amino acid ester of 2(1H)-cytosine, its pharmaceutically acceptable salt and its preparation method. Background technique: The chemical name of the compound commonly known as cytarabine is 1-β-D-arabinofuranosyl-4-amino-2(1H)-cytosine. Cytarabine is converted into active cytarabine triphosphate in the body. Cytarabine triphosphate inhibits DNA polymerase and penetrates into DNA in a small amount, prevents DNA synthesis and inhibits cell growth. Clinically, cytarabine mainly For the treatment of acute myeloid leukemia. However, the molecular polarity of cytarabine is very large, which leads to poor membrane permeability of the small intestine, so the poor membrane permeability makes the oral bioavailability of cytarabine very low (about 20%)....
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07H19/09C07H1/00A61K31/7068A61P35/02
Inventor 何仲贵孙勇兵孙进施世良许佑君张天虹
Owner SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com