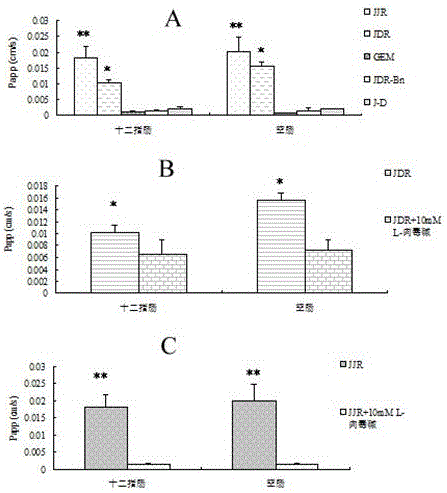
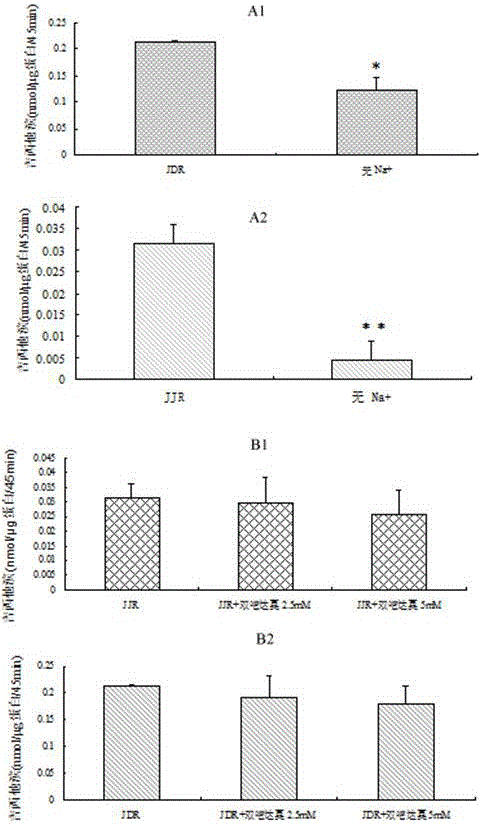
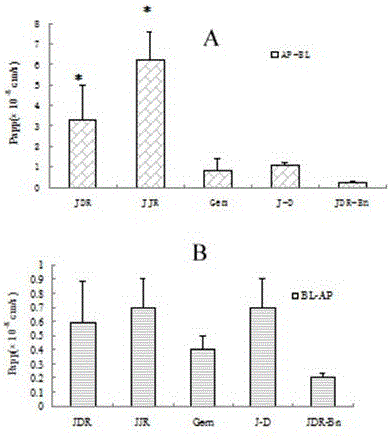
Prodrug based on intestinal OCTN2 carrier protein design and preparation method thereof
A prodrug and pharmaceutical technology, applied in the field of medicine, can solve the problems of low oral bioavailability and poor permeability, and achieve the effect of increasing compliance and reducing risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 14
[0039] Preparation of Example 14-Benzyl L-carnitine
[0040] Dissolve 5.3g (32.9mmol) of L-carnitine and 4.50g (26.3mmol) of bromide in 20mL of DMF, heat to reflux at 140°C for 2h, and concentrate under reduced pressure to obtain 5.53g of white solid with a yield of 83.3%. ESI-MS[M+H] + (m / z): 252 (Intermediate 1).
example 2
[0041] Example 22-butyryl-4-benzyl-L-carnitine preparation
[0042] Dissolve 1g (3.9mmol) of the intermediate and 0.48g (4.8mmol) of succinic anhydride in 10mL of DMF, add 2 drops of triethylamine dropwise at 0°C, and vacuum nitrogen protection after dropping, and the reaction is complete after 2 hours. Processing proceeds directly to the next step. ESI-MS[M+H] + (m / z): 352 (Intermediate 2).
example 32
[0043] Preparation of Example 32-hexanoyl-4-benzyl-L-carnitine
[0044] Dissolve 1g (3.9mmol) of the intermediate and 0.59g (4.8mmol) of adipic anhydride in 10mL of DMF, add 2 drops of triethylamine dropwise at 0°C, and vacuum the nitrogen protection after dropping. After 2 hours, the reaction is complete, and no treatment is required for the reaction Proceed directly to the next step. ESI-MS[M+H] + (m / z): 380 (Intermediate 3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com