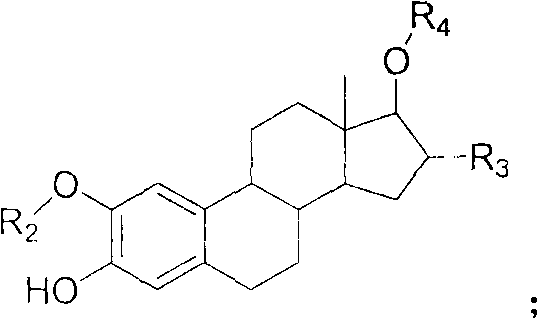
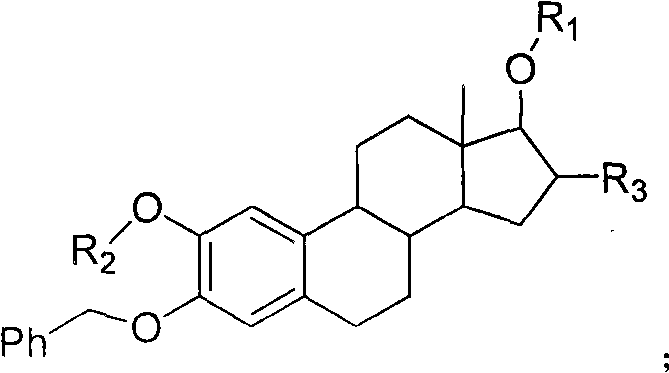
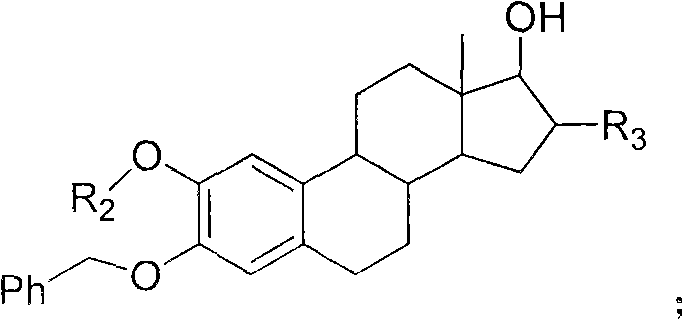
Estrogen amino-acid ester compound with antitumor activity as well as synthetic method thereof
A technology of steroidal amino acid ester and anti-tumor activity, applied in the field of medicine, can solve the problems of poor water solubility, low bioavailability, and restrictions on wide application, and achieve the effect of good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of 2-methoxy-3,17β-dihydroxy-1,3,5(10)-estratriene-17-β-alanine ester
[0035] (1), the preparation of N-benzyloxycarbonyl-beta-alanine
[0036] At 5°C, 10 mmol of β-alanine was dissolved in 4.0 ml of 10% sodium hydroxide (NaOH) aqueous solution to obtain a solution, and 14 mmol (i.e. 1.8 ml) of benzyl chloroformate was added while stirring. Add dropwise to the solution solution at a speed of 1 drop / 4 seconds for reaction, and add dropwise a 10% sodium hydroxide (NaOH) aqueous solution to keep the pH at 8. After the drop is complete, place it at 10°C and stir for 15 minutes. After 1 hour, use ninhydrin as the color developing agent to develop the color without purple color, then wash with 5ml / time ether for 3 times, discard the ether to obtain the aqueous phase, and adjust the pH of the aqueous phase to 3 with concentrated hydrochloric acid with a mass concentration of 30%. A milky white solid appeared, and the liquid after the milky white solid ...
Embodiment 2
[0041] Example 2: Preparation of 16α-ethyl-2-methoxy-3,17β-dihydroxy-1,3,5(10)-estratriene-17-L-phenylalanine ester
[0042] (1), the preparation of N-benzyloxycarbonyl-L-phenylalanine
[0043] At -3°C, 10 mmol of L-phenylalanine was dissolved in 4.0 ml of 9% sodium hydroxide (NaOH) aqueous solution to obtain a solution, and 14 mmol (i.e. 1.8 ml) of chloroformic acid was added while stirring. The benzyl ester is added dropwise to the solution at a speed of 1 drop / 5 seconds for reaction, and a 9% sodium hydroxide (NaOH) aqueous solution is added dropwise to keep the pH at 8. After the drop is completed, place it at 15°C and stir React for 15 hours, do TLC detection, after the solution is completely reacted, wash 3 times with 5ml / time of diethyl ether, discard the diethyl ether to obtain the water phase, and adjust the pH of the water phase to 2 with concentrated hydrochloric acid with a mass concentration of 35%. , a milky white solid appeared, and the liquid after the milky w...
Embodiment 3
[0048] Example 3: 16α-(N,N-dimethyl)aminomethyl-2-methoxy-3,17β-dihydroxy-1,3,5(10)-estratriene-17-L-leu Preparation of Amino Acid Esters
[0049] (1), the preparation of N-benzyloxycarbonyl-L-leucine
[0050] At 2°C, 10 mmol of L-leucine was dissolved in 4.0 ml of 8% sodium hydroxide (NaOH) aqueous solution to obtain a solution, and 14 mmol (i.e. 1.8 ml) of benzyl chloroformate was added while stirring. Add dropwise to the solution solution at a speed of 1 drop / 6 seconds for reaction, and add dropwise an aqueous solution of sodium hydroxide (NaOH) with a mass concentration of 8% to keep the pH at 9. After the drop is complete, place it at 20°C and stir for 15 minutes. After 1 hour, use ninhydrin as the color developing agent to develop the color without purple color, then wash with 5ml / time of diethyl ether for 3 times, discard the diethyl ether to obtain the aqueous phase, and adjust the pH of the aqueous phase to 1 with concentrated hydrochloric acid with a mass concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com