Preparation method of Nedocromil sodium
A dihydro and ethyl technology, applied in the field of preparation of nedocromil sodium, can solve the problems of low actual yield, short steps, incomplete breakage of ether bonds, etc., and achieve the effect of increasing the synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
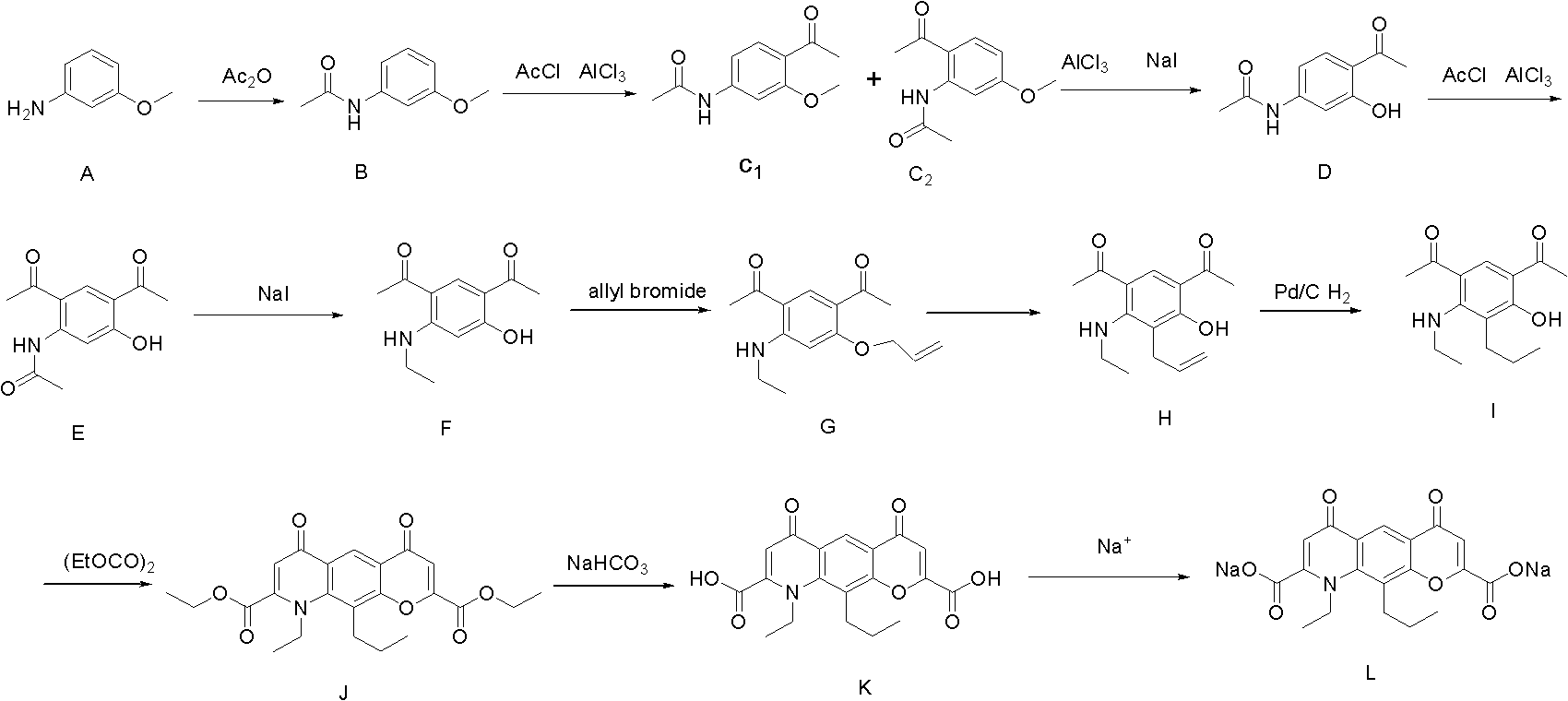
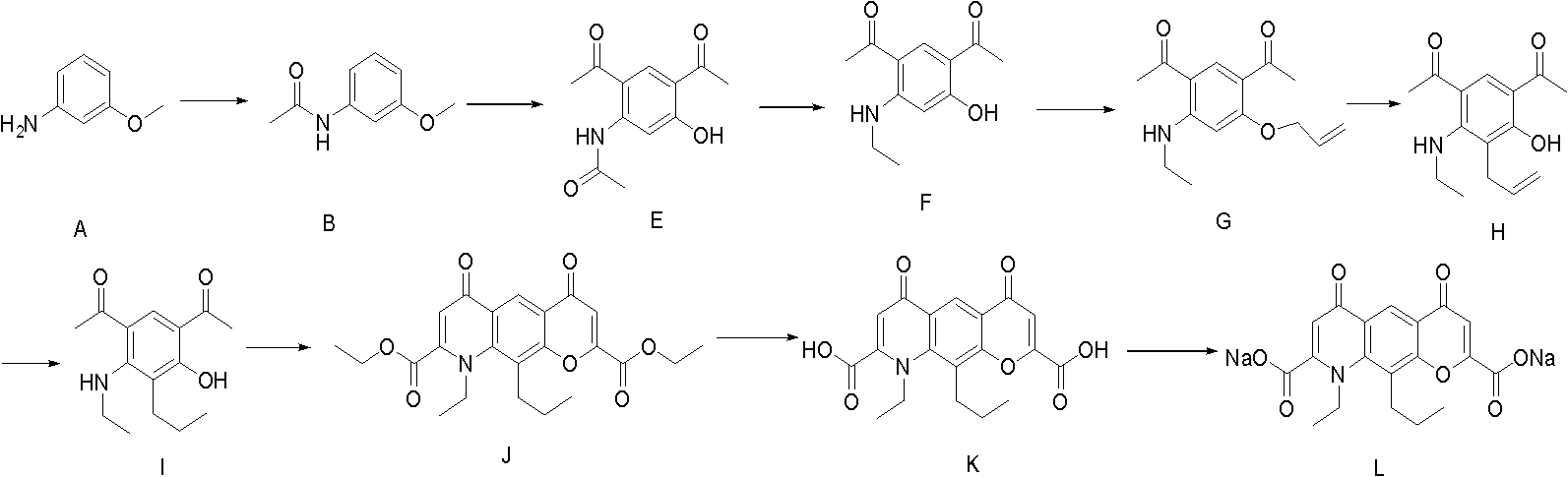
[0037] Example 1 Preparation of 3-methoxyacetanilide (compound B)
[0038]
[0039] In a 2000ml round-bottomed three-neck flask, add 700g of the raw material compound A m-aminoanisole, stir mechanically, and add 600ml of glacial acetic acid dropwise at about 5°C. After the addition, stir for 10 minutes, and add 595ml of acetic anhydride dropwise at the same temperature. After dropping, continue to stir for 10 min, rise to 20°C and react for 2 h. Below room temperature, the reaction solution was slowly poured into the lye solution made of 800g sodium hydroxide and 3L water, and a white solid was precipitated. Filter, wash with water, and dry in vacuo to obtain 890 g of off-white solid 3-methoxyacetanilide (Compound B), yield: 94%, melting point: 77-80°C.
Embodiment 2
[0040] Example 2 3-methoxyl group-4-acetyl-acetanilide (compound C 1 ) preparation
[0041]
[0042] In a 5000ml three-necked bottle, add 860g of aluminum trichloride, protect it with argon, add 1.2L of dichloromethane, stir mechanically, add 473ml of acetyl chloride dropwise at around 0°C, and then add 350g of 3-methoxyl dropwise at the same temperature A mixed solution of acetanilide (compound B) and 2.3L of dichloromethane was added, and reacted at about 0°C for 4 hours after dropping. After the reaction is complete, the reaction solution is carefully poured into 4L of ice water below room temperature, extracted with dichloromethane, and concentrated in vacuo to obtain light yellow 3-methoxy-4-acetyl-acetanilide (compound C1) and isomer 3-methanilide. Oxy-6-acetyl-acetanilide (compound C2) mixture 423g, yield 96.3%
Embodiment 3
[0043] Example 3 Preparation of 3-acetamido-4-acetylphenol (compound D)
[0044]
[0045] In a 5000ml three-necked flask, add 423g of a mixture of 3-methoxy-4-acetyl-acetanilide (compound C1) and isomer 3-methoxy-6-acetyl-acetanilide (compound C2), acetonitrile 4000ml, stirred until completely dissolved, protected by argon, added 600g of anhydrous aluminum trichloride in batches below 15°C, quickly added 400g of sodium iodide at around 0°C, raised the temperature to 20°C, and stirred for 15h. After the reaction was completed, the reaction solution was poured into 7 L of water dissolved with 380 g of sodium thiosulfate, extracted with ethyl acetate to obtain an organic layer, and the organic layer was back-extracted with 20% aqueous sodium hydroxide solution. The lye layer was obtained, and concentrated hydrochloric acid was used to adjust the pH of the lye layer to 3-4. A large amount of white solids were formed. After suction filtration, 388 g of light yellow solid 3-aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com