Preparation method for 4-aminoacetophenone
An aminoacetophenone, hydroxyl technology, applied in a new preparation field of p-aminoacetophenone, can solve the problems of harsh reaction conditions, many by-products, strong reagent corrosion and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the preparation of N-(4-acetylphenyl)-2-hydroxyl-2-methylpropionamide
[0019] Dissolve 3.00g (22.06mmol) of p-hydroxyacetophenone in 15mL of DMA in a 100mL round bottom flask, add 2.65g (66.18mmol) of sodium hydroxide and stir the reaction at 15-25°C for 1 hour. Subsequently, 11.00 g (66.18 mmol) of 2-bromo-2-methylpropanamide was added and the reaction was stirred at 15-25°C for 5 hours (the product of this step was directly carried to the next step without isolation). After the reaction, 7.94g (198.54mmol) sodium hydroxide was added and stirred at 45-50°C for 1 hour, and finally 30mL of water was added and stirred at room temperature for crystallization. After suction filtration and natural air drying, 2.90 g of yellow crystal powder was obtained with a yield of 59.5%, which can be directly used in the next reaction without further purification.
[0020] Similarly, substitute 2-chloro-2-methylpropanamide for 2-bromo-2-methylpropanamide, potassium hydro...
Embodiment 2
[0027] Embodiment 2: the preparation of p-aminoacetophenone
[0028] Dissolve 4.72 g (118.08 mmol) of sodium hydroxide in 13 mL of water in a 100 mL round bottom flask, then add 13 mL of DMA and 2.90 g (13.12 mmol) of N-(4-acetylphenyl)-2-hydroxy- 2-methylpropanamide, and heated to reflux at 90°C for 1 hour. After the reaction was completed, 26 mL of water was added and stirred at room temperature for crystallization. After suction filtration and natural air drying, 1.55 g of yellow crystal powder was obtained with a yield of 87.6% and a total yield of 52.1%.
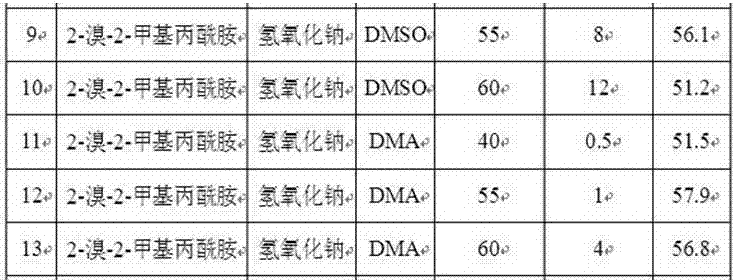
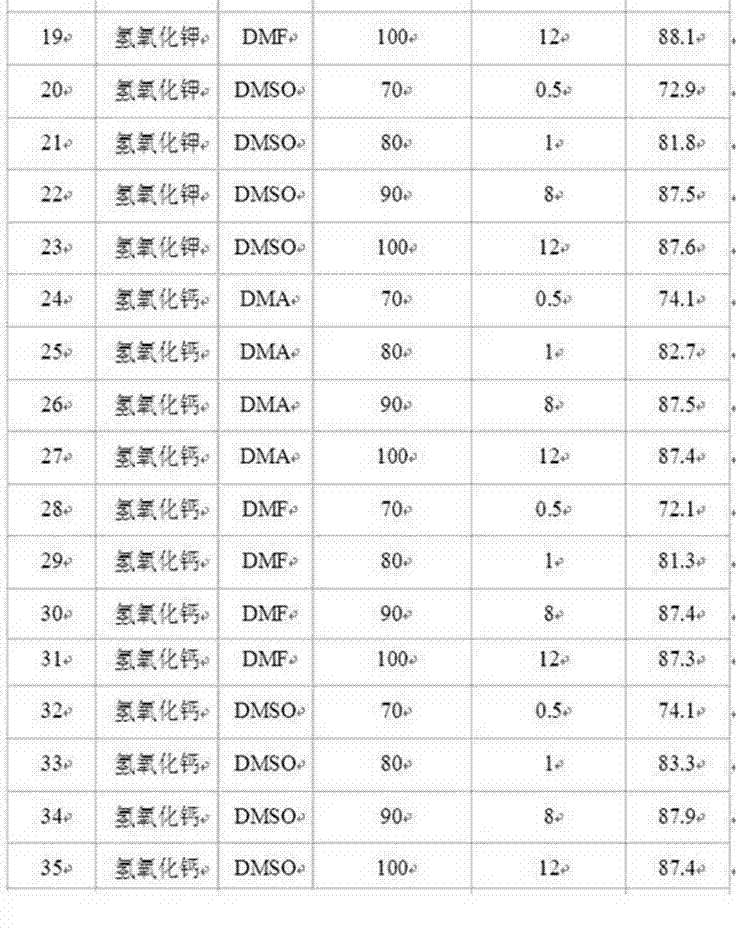
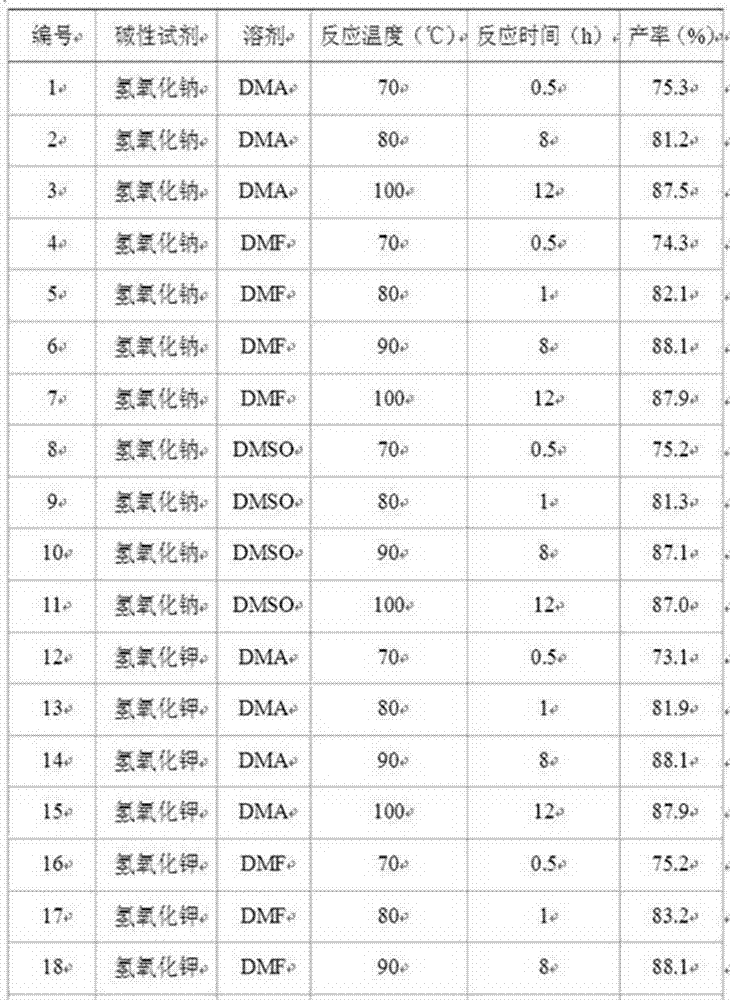
[0029] Similarly, replace sodium hydroxide with potassium hydroxide or calcium hydroxide, and replace N,N-dimethylacetamide (DMA) with N,N-dimethylformamide (DMF) or dimethylsulfoxide (DMSO). ), change the hydrolysis reaction temperature and reaction time to obtain p-aminoacetophenone, and the results are listed in Table 2.
[0030]
[0031] Table 2. Preparation of p-aminoacetophenone
[0032]
[0033]
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com