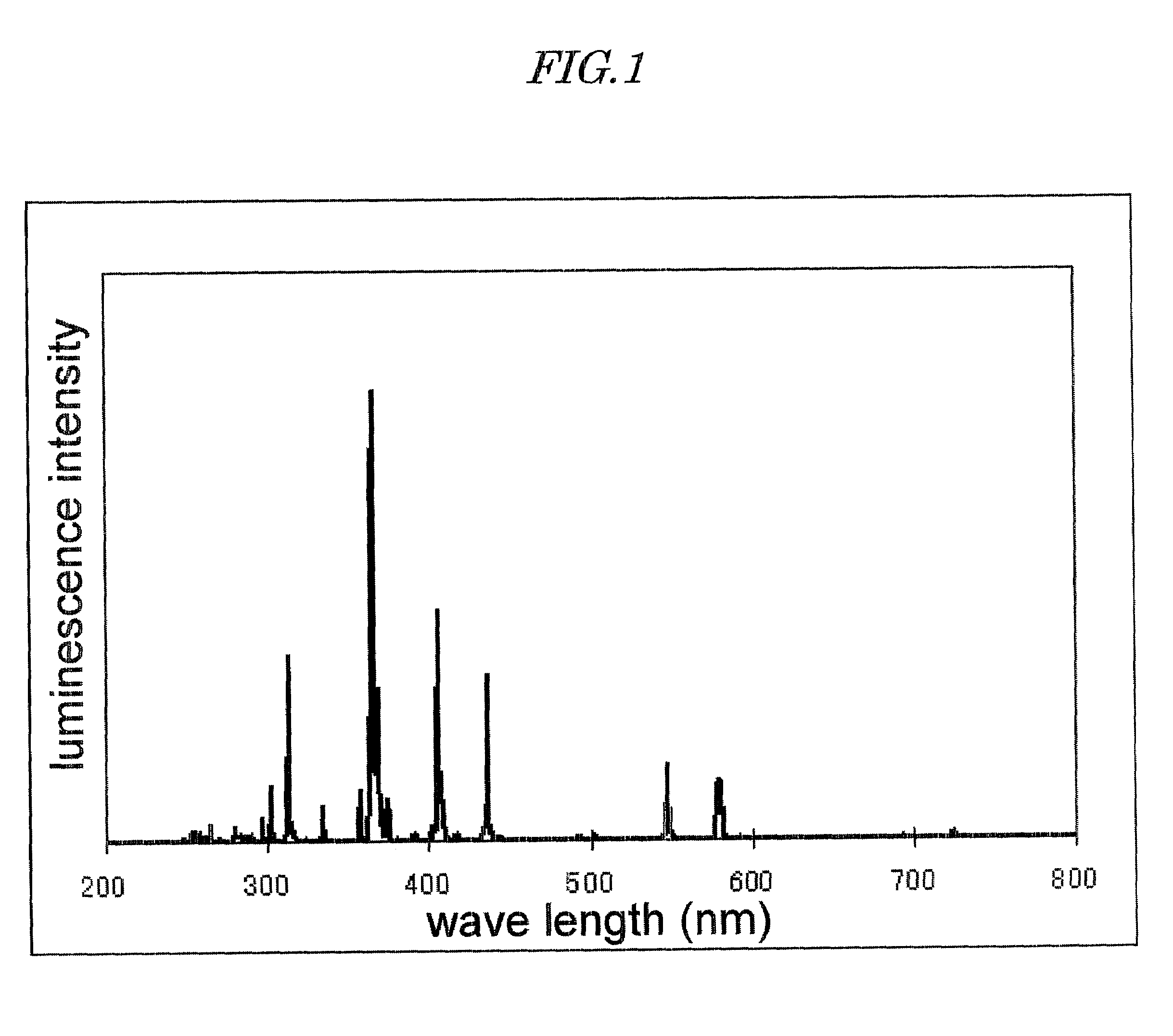
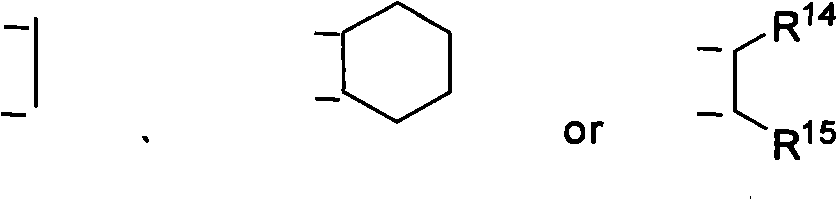
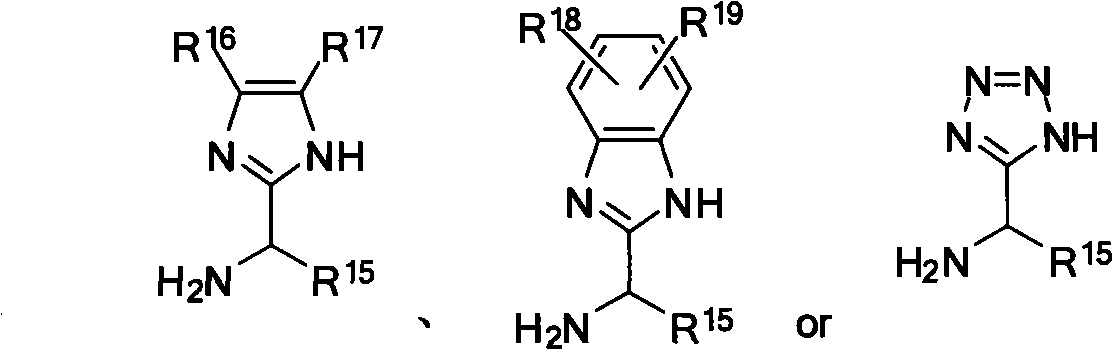
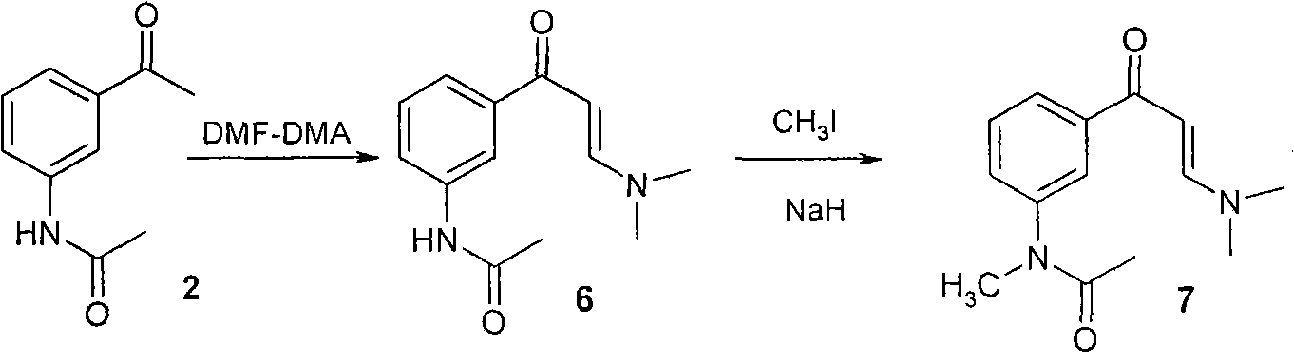
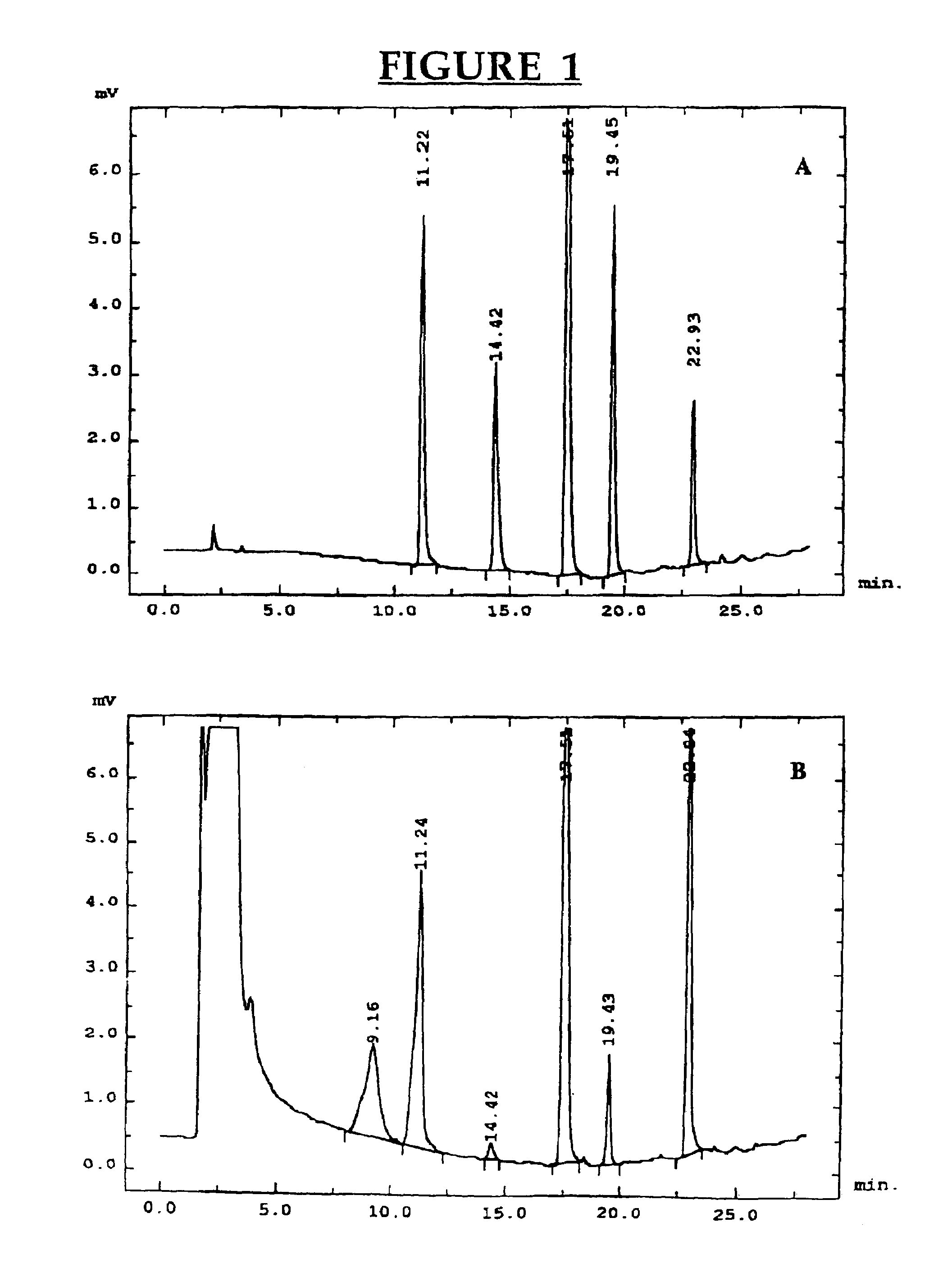
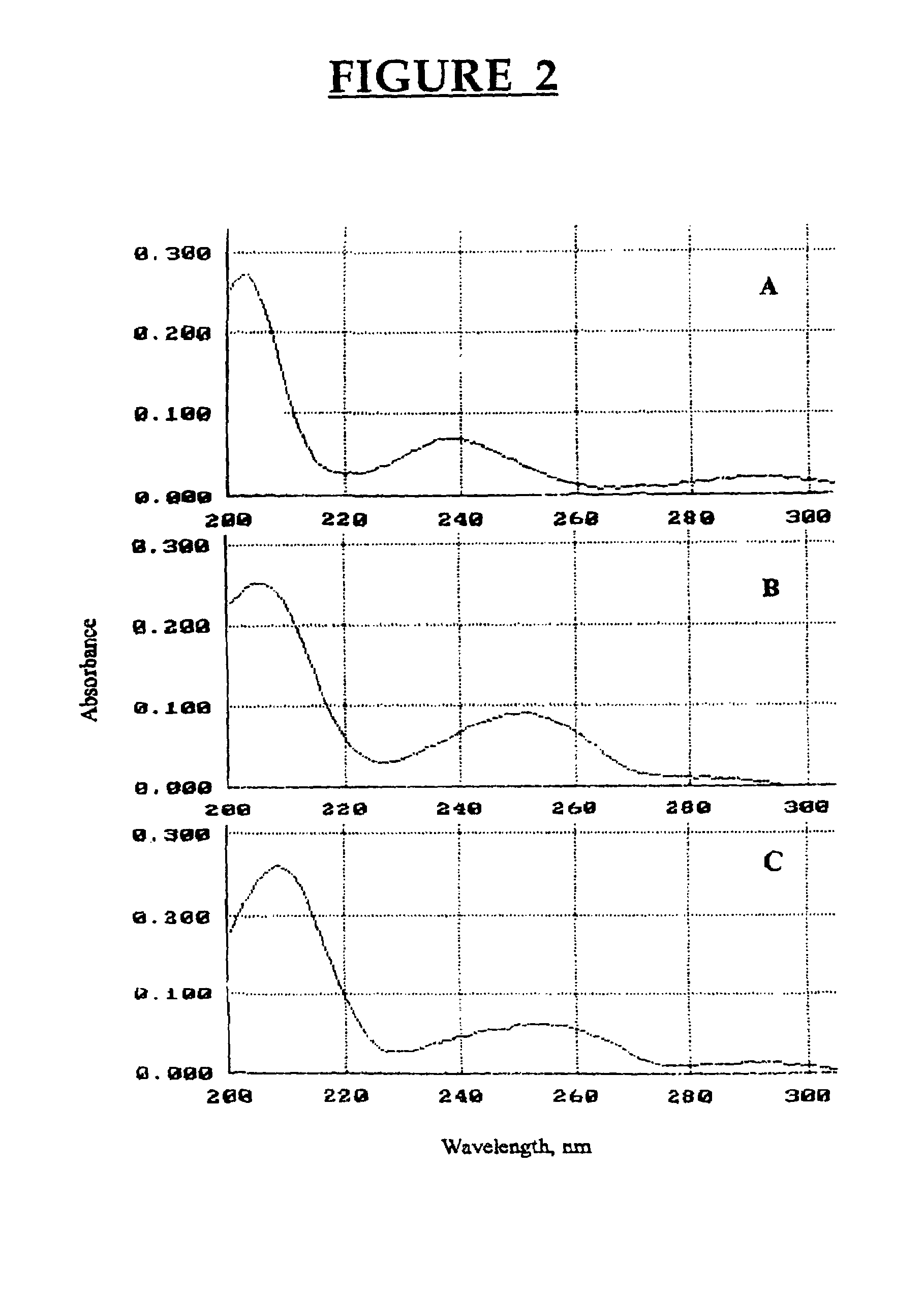
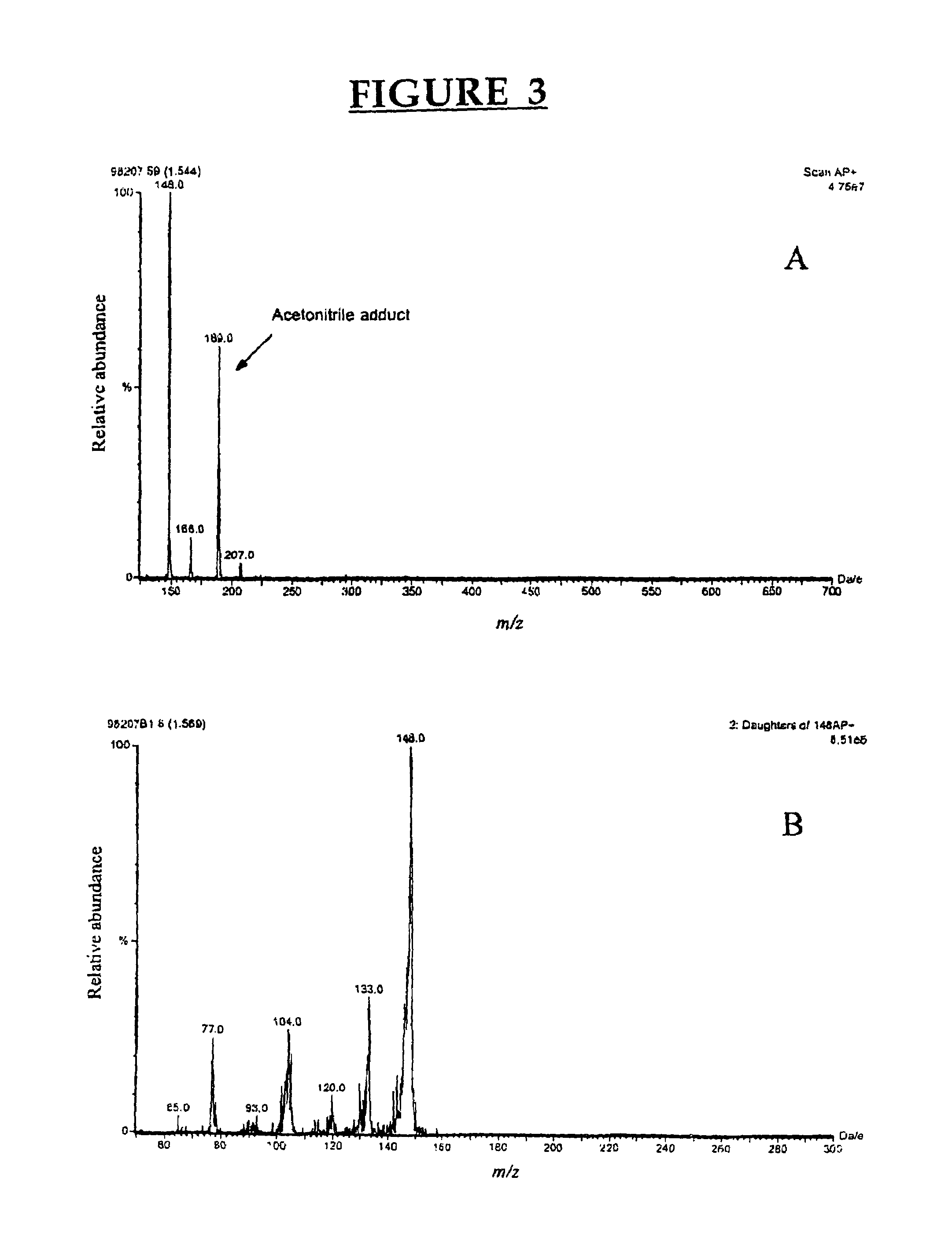
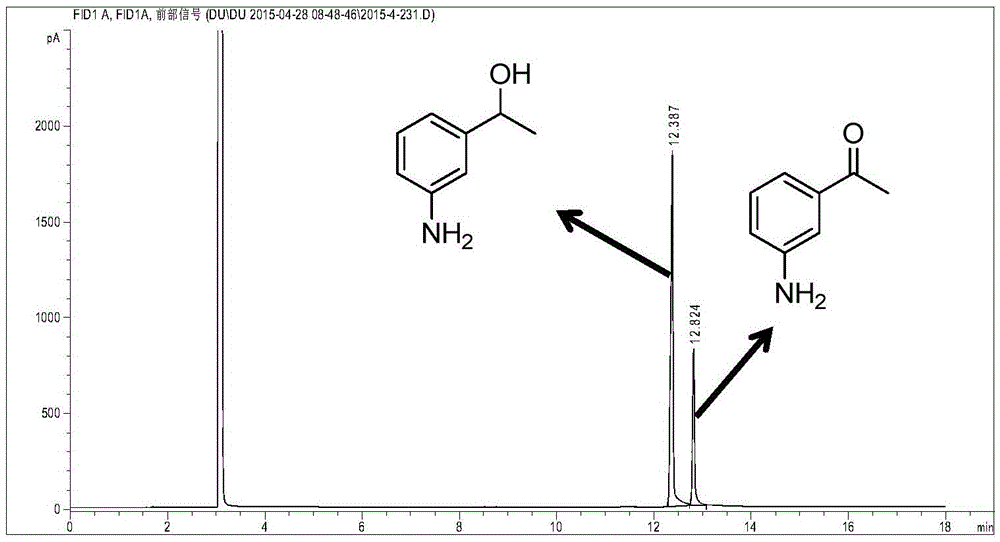
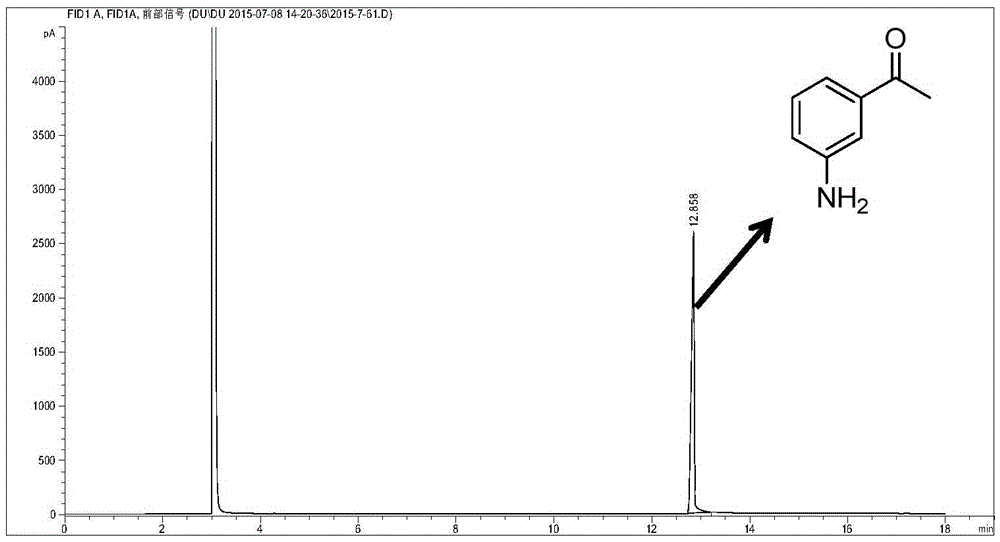
Patents
Literature
90 results about "M-aminoacetophenone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
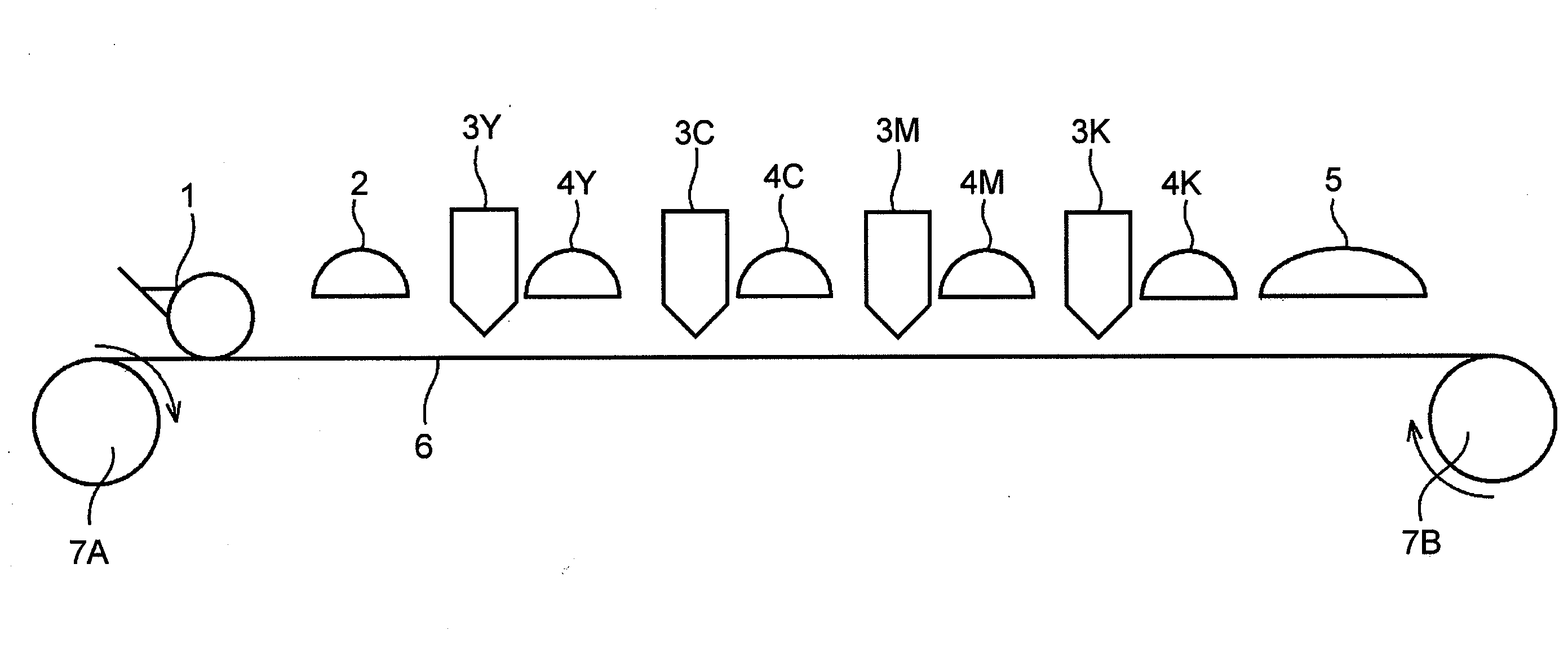
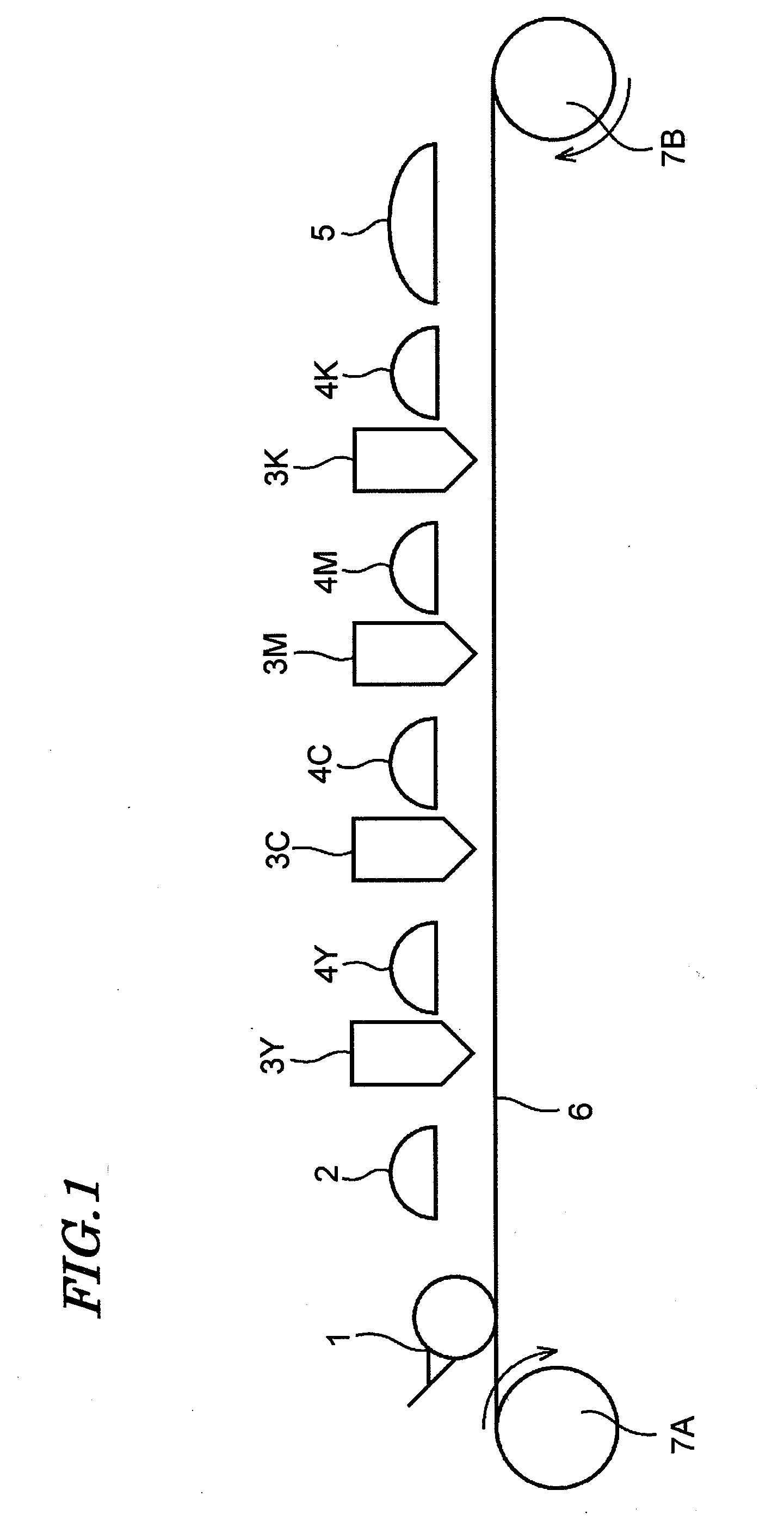
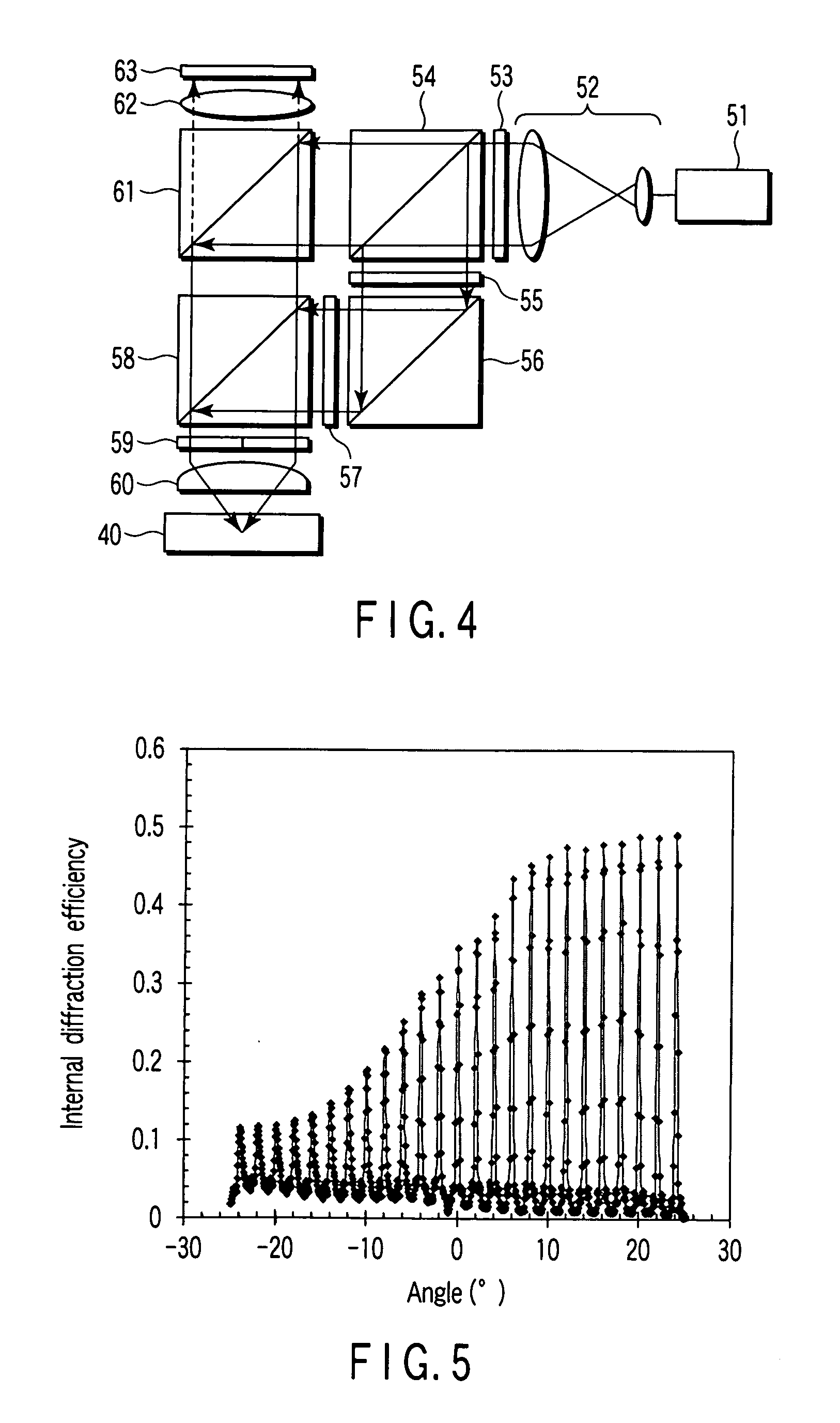
Ink set for inkjet recording and inkjet recording method
ActiveUS20080180503A1Inhibited DiffusionGood image uniformityMeasurement apparatus componentsInksM-aminoacetophenoneImage formation
An ink set for inkjet recording is provided that includes at least a colored liquid composition comprising at least a radically polymerizable compound, a photopolymerization initiator, and a colorant, and an undercoat liquid composition comprising at least a radically polymerizable compound and a photopolymerization initiator, the colored liquid composition comprising as the photopolymerization initiator at least one type of α-aminoacetophenone compound, and the undercoat liquid composition comprising as the photopolymerization initiator at least one type of compound selected from the group consisting of an acylphosphine oxide compound, an α-hydroxyacetophenone compound, and an oxime ester compound. There is also provided an inkjet recording method employing the ink set for inkjet recording, the method including a step of applying the undercoat liquid composition on top of a recording medium, a step of semi-curing the undercoat liquid composition, and a step of carrying out image formation by discharging the colored liquid composition on top of the semi-cured undercoat liquid composition.
Owner:FUJIFILM CORP
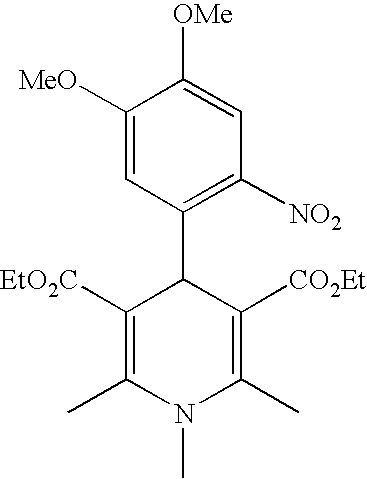
Photocurable composition, photocurable ink composition, process for producing photocured material, and inkjet recording method
InactiveUS20090186163A1High sensitivityLittle discolorationConductive materialInksM-aminoacetophenoneHydrogen atom
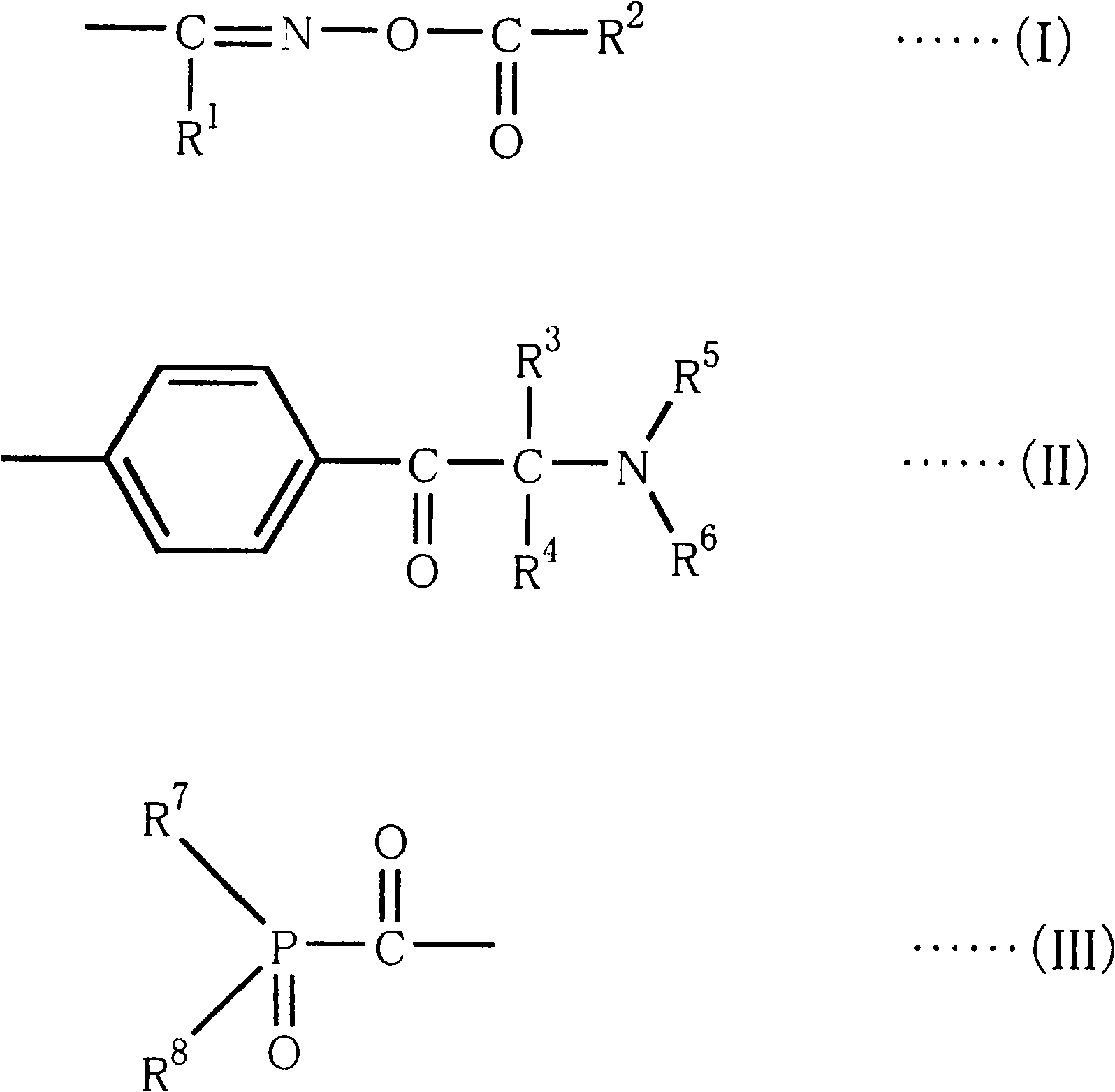
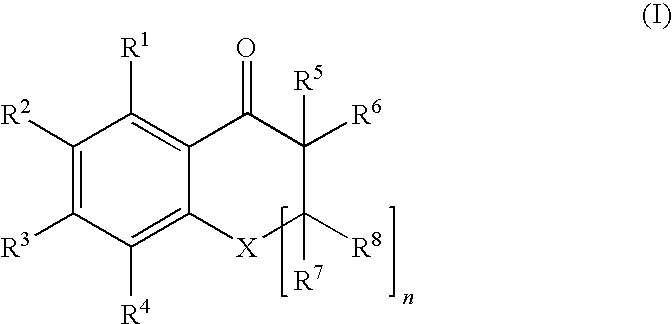
A photocurable composition is provided that includes a polymerizable compound, a photopolymerization initiator, a compound represented by Formula (I) below, and an amine compound, the photopolymerization initiator comprising an acylphosphine oxide compound and / or an α-aminoacetophenone compoundin Formula (I) above, X denotes O, S, or NR, n denotes 0 or 1, R denotes a hydrogen atom, an alkyl group, or an acyl group, R1, R2, R3, R4, R5, R6, R7, and R8 independently denote a hydrogen atom or a monovalent substituent, and two of R1, R2, R3, and R4 that are adjacent may be bonded to each other to form a ring. There are also provided a photocurable ink composition that includes the photocurable composition and a colorant, an ink composition for photocuring inkjet recording that includes the photocurable ink composition, a process for producing a photocured material that includes preparing the photocurable composition and irradiating the photocurable composition with light having a light emission peak in the range of at least 340 nm but no greater than 400 nm, and an inkjet recording method that includes discharging the ink composition onto a recording medium and irradiating the ink composition with actinic radiation.
Owner:FUJIFILM CORP
Nitrogen-containing ligand transient metal complex compound , synthetic method and use thereof
The invention relates to a nitrogenous ligand transition metal complex, a method for synthesizing the same and application of the same, in particular to a metal ruthenium complex with a structural formula shown on the right, other transition metal complexes, a method for synthesizing the same and an application of the same. A diphosphine and dinitrogen transition metal complex which is formed by coordination between a dinitrogen ligand with structural characteristics of NH2-N(sp<2>) and a transition metal can be used for catalytic asymmetrical transfer hydrogenation reaction and also can be used for catalytic hydrogenation reaction, and particularly used for asymmetric catalytic hydrogenation reaction of ketone with large steric hindrance alkyl on the alpha position, hypnone, hypnone derivatives, benzophenone, benzophenone derivatives, beta-N, N-dimethyl amino-alpha hypnone, derivatives of the beta-N, N-dimethyl amino-alpha hypnone, and other ketone compounds.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
Photo-cured heat-cured resin composition and printed circuit board produced with the same
ActiveCN101320212AExcellent developabilityAvoid inactivationPhotomechanical apparatusPrinted circuit manufactureM-aminoacetophenoneUltraviolet
A light-curing thermo-curing resin composition and a printed circuit board obtained using the same are provided, the composition has high light sensitivity and excellent curing depth in ultraviolet ray and laser exposure, thus further having great developing ability to pass through diluted alkaline aqueous solution while being excellent in storage stability and operability, so the composition is suitable for solder resisting agent. The light-curing thermo-curing resin composition comprises (A) carboxyl-containing resin, (B) mercaptobutyric acid or derivatives thereof, (C) photopolymerization initiator, (D) compounds having 2 or more of olefinic unsaturated groups in the molecules, and (E) thermo-curing components. The suitable (A) carboxyl-containing resin is preferably the carboxyl-containing resin capable of free radical polymerization and having unsaturated double bond. In addition, the (C) photopolymerization initiator is preferably oximes photopolymerization initiator (C1), particularly preferably proxetil photopolymerization initiator, aminoacetophenone photopolymerization initiator and / or acyl phosphine oxide photopolymerization initiator.
Owner:TAIYO HLDG CO LTD
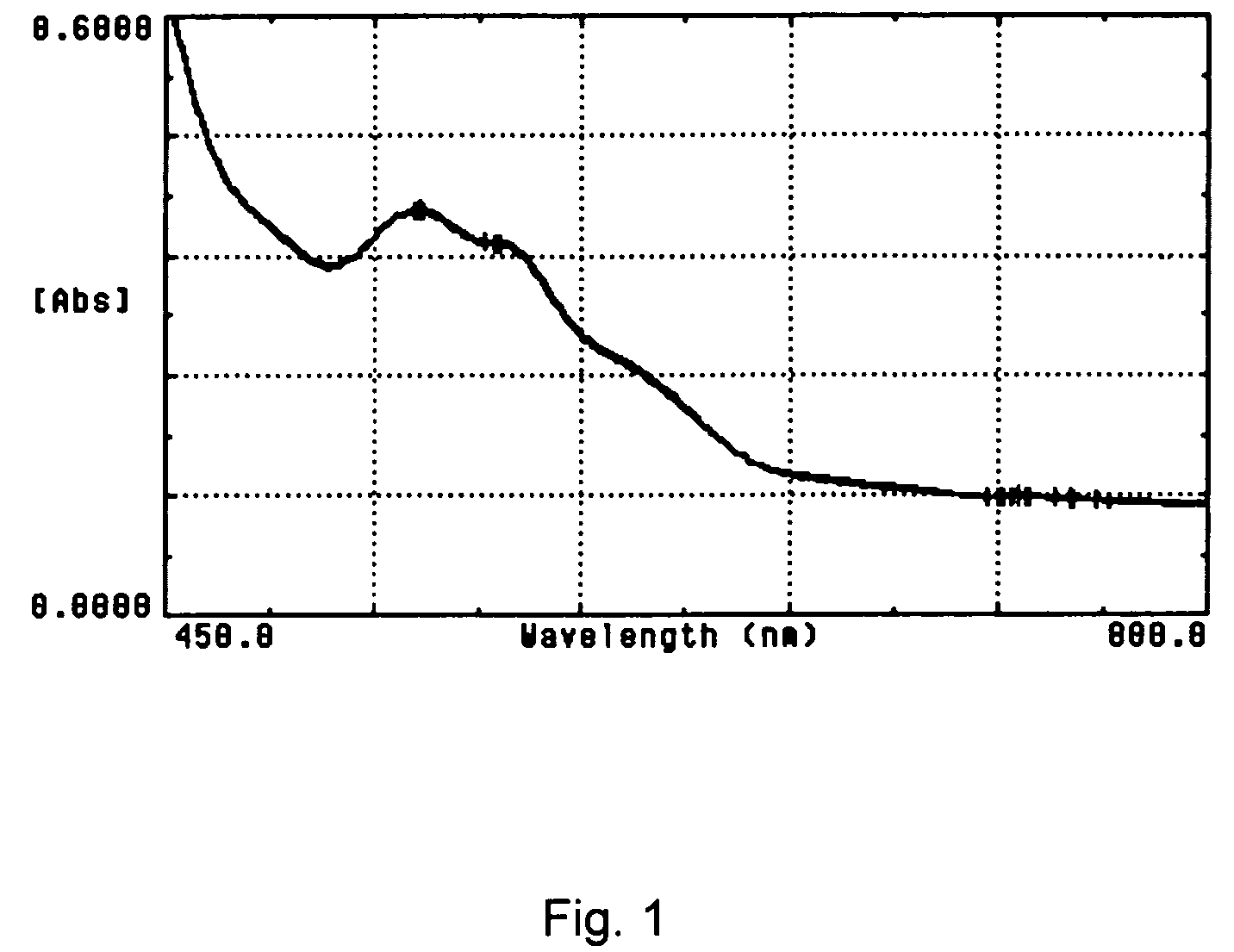
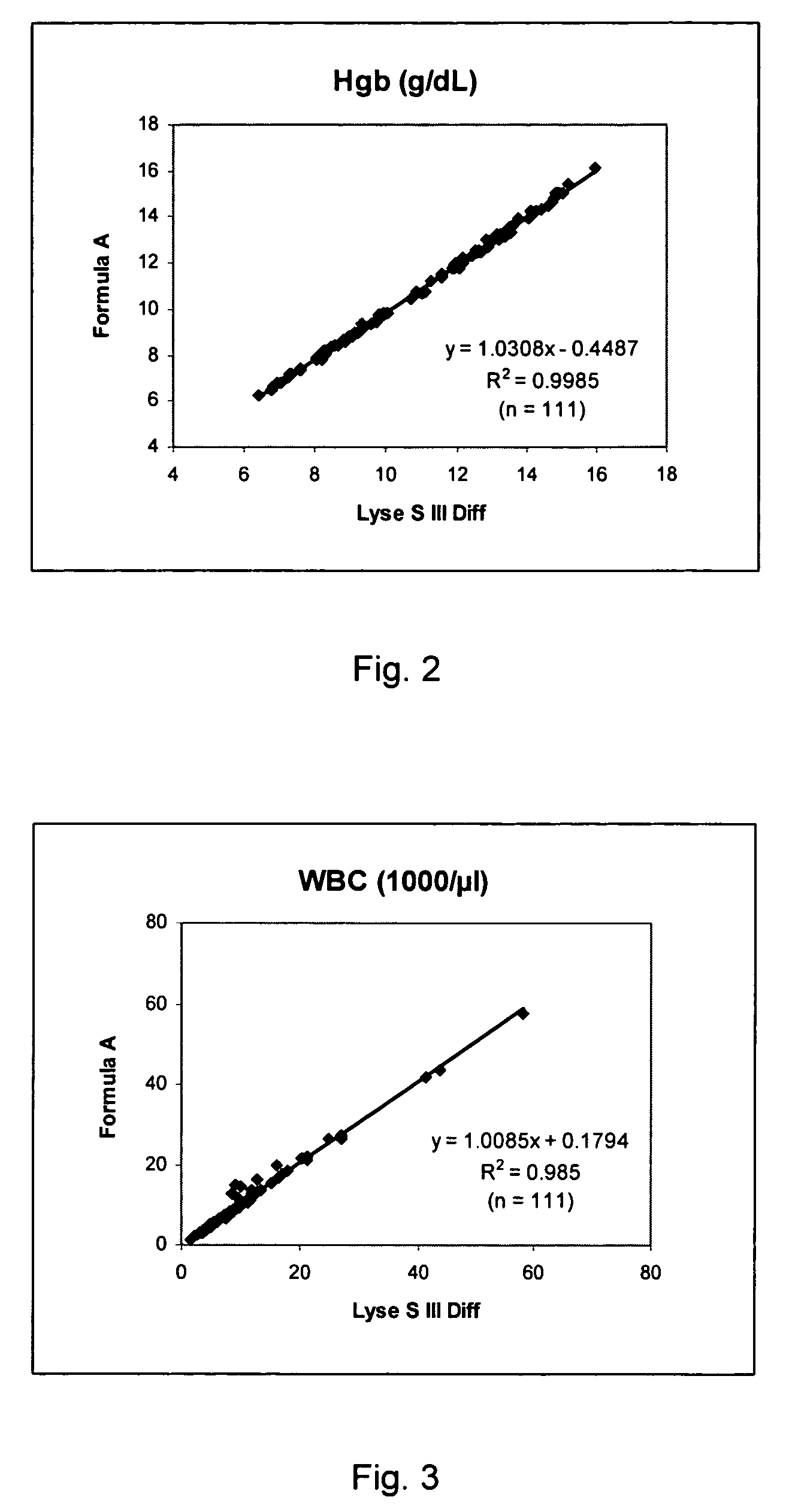
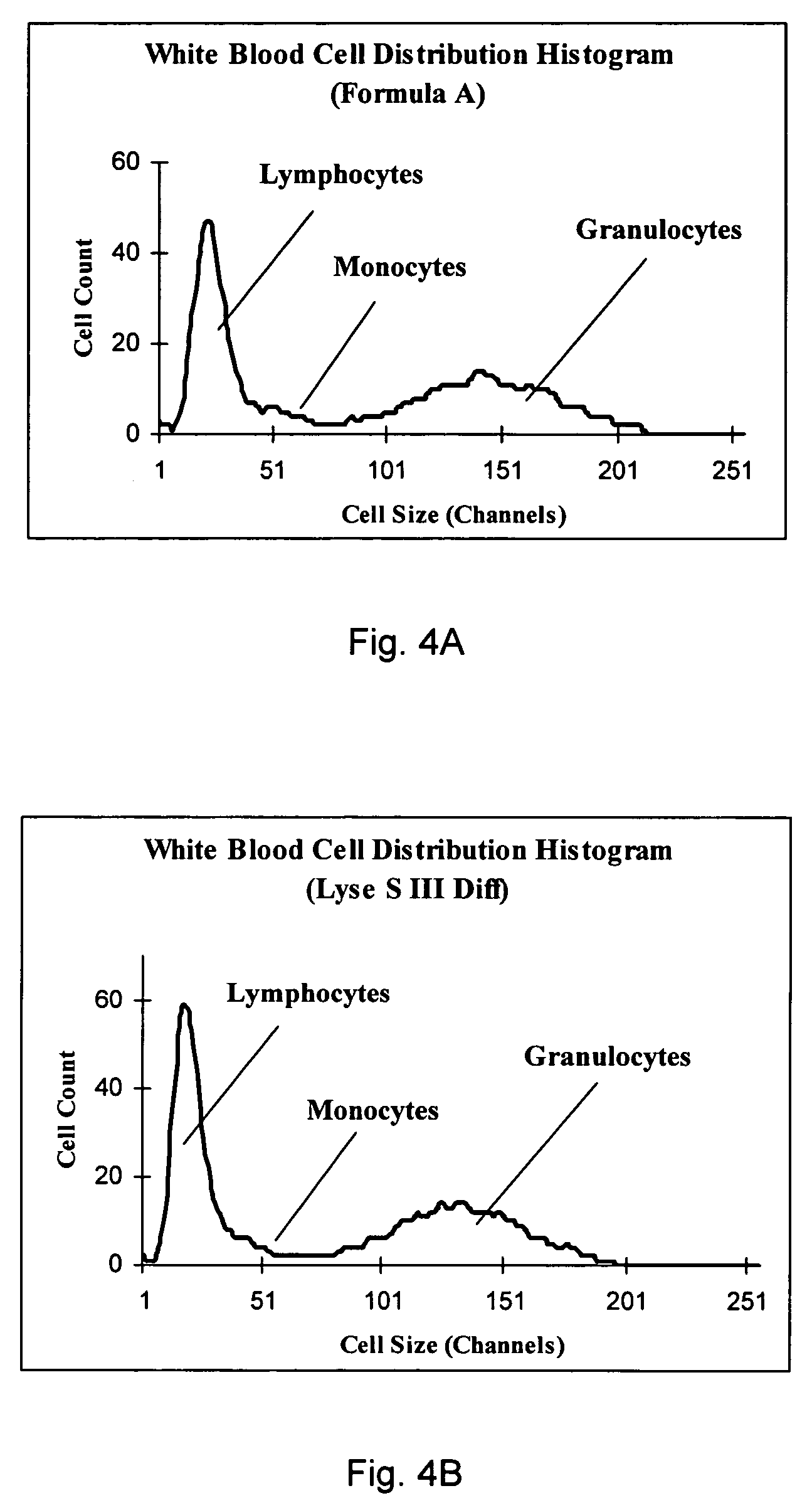
Cyanide-free lytic reagent composition and method of use for hemoglobin and white blood cell measurement
A cyanide-free lytic reagent composition and method of use for measuring the total hemoglobin concentration and white blood cells in a blood sample are disclosed. The lytic reagent composition includes a quaternary ammonium surfactant to lyse red blood cells and release hemoglobin and a ligand to form a stable chromogen with the hemoglobin. The lytic reagent composition has a pH in a range of 3 to 10. The lytic reagent composition can also include a second quaternary ammonium surfactant. The ligand can be malic acid, malonic acid, ethylene diamine, N,N-diethylethylene diamine, N,N′-diethylethylene diamine, diethylene triamine, tetraethylene pentamine, 1,6-hexanediamine, 1,3-pentanediamine, 2-methylpentamethylenediamine, 1,2-diaminocyclohexane, 4-aminoacetophenone, bis-hexamethylenetriamine, pyridazine, or 3,6-dihyroxypyridazine.
Owner:BECKMAN COULTER INC
Photocurable and thermosetting resin composition, cured product thereof and printed circuit board obtained using the same
InactiveUS20080096133A1Excels in photo-curing propertySuitable for usePhotosensitive materialsPhotomechanical apparatusM-aminoacetophenonePolymer science
A photocurable and thermosetting resin composition comprising (A) a carboxyl-group containing resin, (B) an oxime ester-based photopolymerization initiator containing an oxime ester group, (C) an aminoacetophenone-based photopolymerization initiator and / or a phosphine oxide-based photopolymerization initiator, (D) a compound having at least two ethylenically unsaturated groups in its molecule, (E) a filler, and (F) a thermosetting component is capable of being developed with a dilute alkaline solution and cured with a laser emission source having a maximum wavelength of 350 nm to 420 nm.
Owner:TAIYO INK MFG
Holographic recording medium
InactiveUS20070030541A1Photosensitive materialsPhotomechanical apparatusM-aminoacetophenoneCompound (substance)
A holographic recording medium has a recording layer including a radical-polymerizable compound, a first photo-radical polymerization initiator (A) containing a titanocene compound, and a second photo-radical polymerization initiator (B) containing at least one compound selected from the group consisting of an amino acetophenone derivative, an acylphosphine oxide derivative, a benzyl derivative, and a thioxanthone derivative.
Owner:KK TOSHIBA
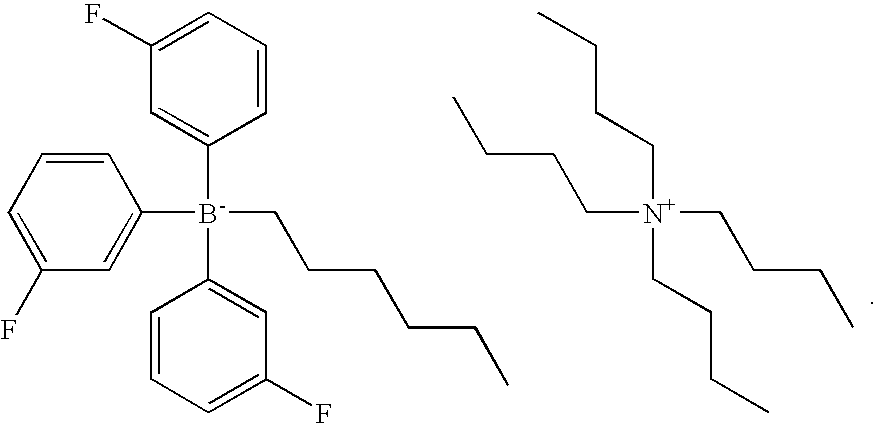
Photoactivatable coating composition
Photoactivatable coating composition comprising at least one photoinitiator and a base-catalysed polymerisable or curable organic material comprising at least one polyisocyanate and at least one compound containing isocyanate reactive groups, wherein the isocyanate reactive groups comprise at least one thiol group and the photoinitiator is a photolatent base.Preference is given to a coating composition wherein the photolatent base is selected from the group of N-substituted 4-(ortho-nitrophenyl)dihydropyridine, a quaternary organo-boron photoinitiator, and an α-amino acetophenone. The composition additionally may comprise an organic acid, a metal complex and / or a metal salt as a cocatalyst and / or a sensitiser selected from the group of thioxanthones, oxazines, rhodamines, and preferably from the group of benzophenone and derivatives thereof.
Owner:AKZO NOBEL NV
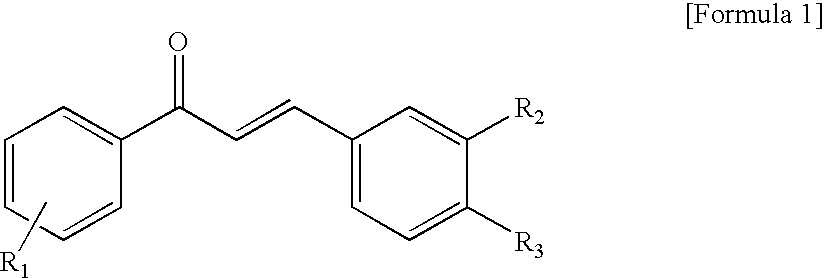
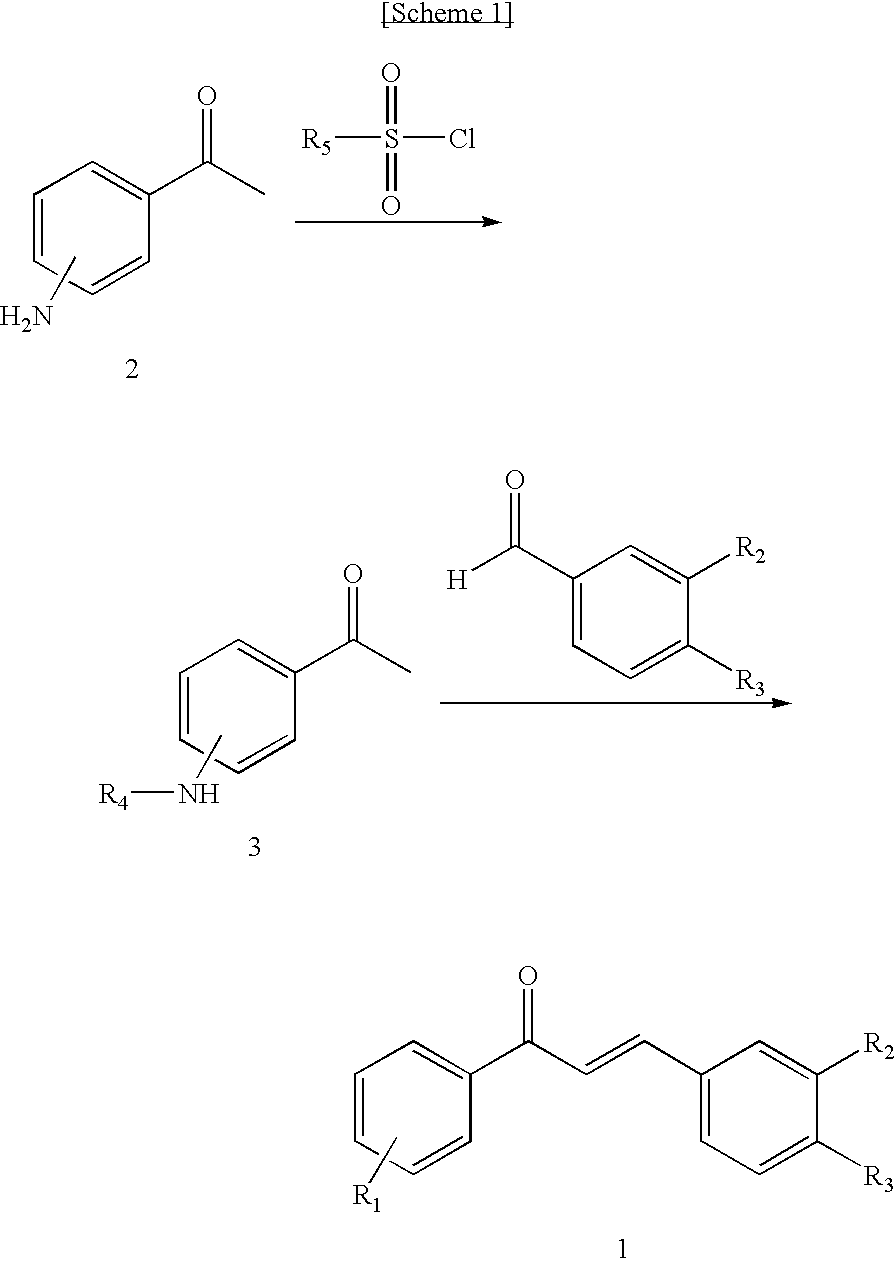
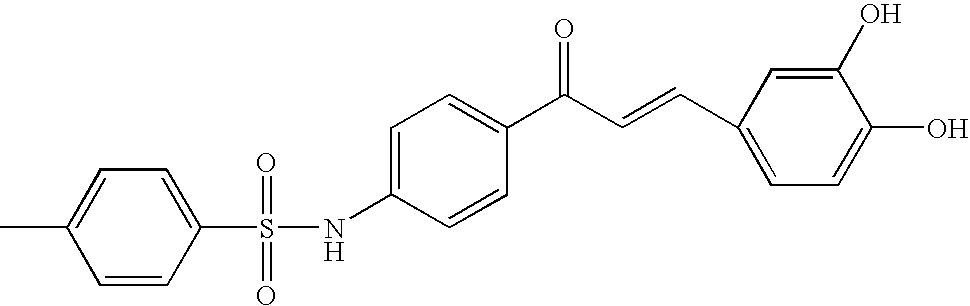
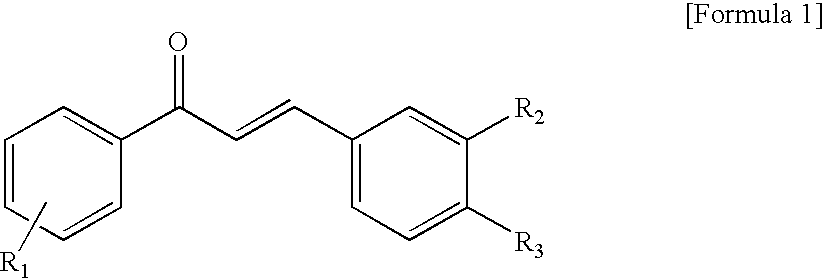
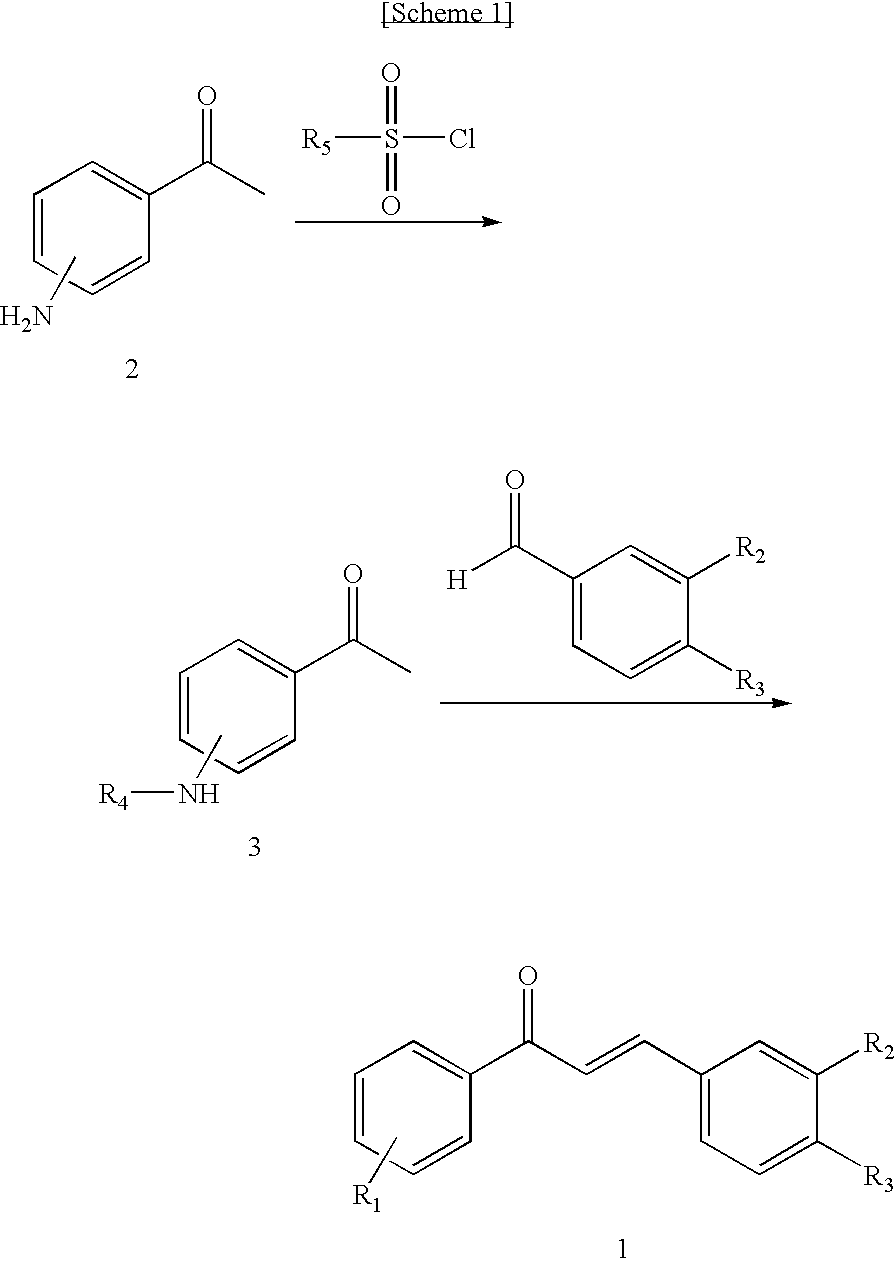
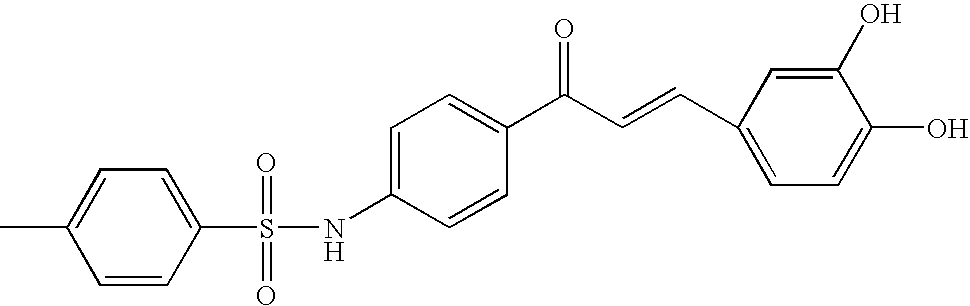
Novel Chalcone Derivatives, Pharmaceutically Acceptable Salt, Method for Preparation and Uses Thereof
InactiveUS20090252694A1Easy to useStrong enzyme inhibitory activityBiocideCosmetic preparationsDiseaseM-aminoacetophenone
Disclosed relates to a novel chalcone derivative, pharmaceutically acceptable salt thereof, a method for preparing the same and uses thereof, the chalcone derivative being readily obtained through the steps of: reacting aminoacetophenone with sulfonylchloride under the presence of an appropriate salt; and reacting the compound prepared in the above step with hydroxybenzaldehide under the presence of an appropriate catalyst. The chalcone derivative of formula 1 in accordance with the present invention having strong enzyme inhibitory activities for glycosidase can be effectively used in preventing and treating various diseases induced by glycosidase, and the chalcone derivative of the invention having tyrosinase and melanin synthesis inhibitory activities can be effectively used as a skin-whitening compound.
Owner:JCN FARM CO LTD
Photocurable composition, photocurable ink composition, process for producing photocured material, and inkjet recording method
A photocurable composition is provided that includes a polymerizable compound, a photopolymerization initiator that is an acylphosphine oxide and / or an alpha-aminoacetophenone, a compound represented by Formula (I) below, and an amine compound. There are also provided a photocurable ink, a process for producing a photocured material, and an inkjet recording method.
Owner:FUJIFILM CORP
Method for synthesizing indiplon
InactiveCN101591338ALower synthesis costMild reaction conditionsOrganic chemistryM-aminoacetophenoneAcetic anhydride
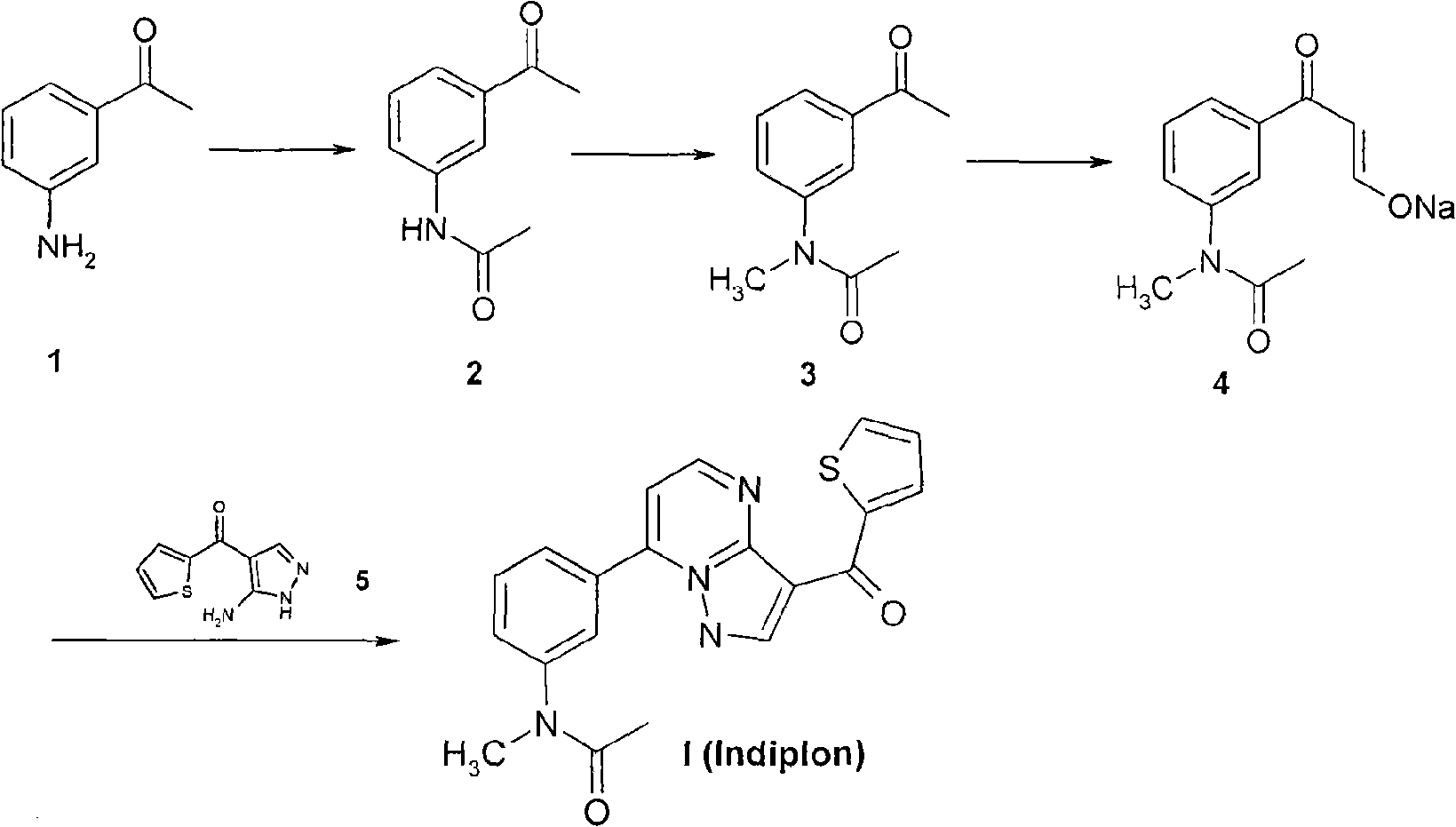
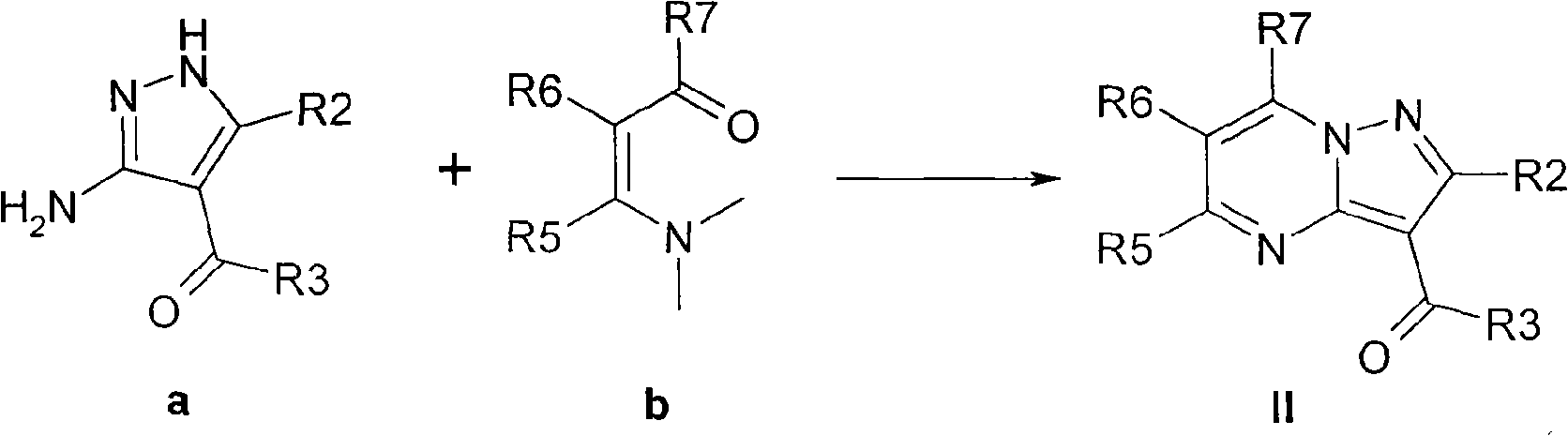
The invention relates to a method for synthesizing indiplon, which comprises the following steps: using low-cost available 3-aminoacetophenone as a starting raw material; reacting the 3-aminoacetophenone with acetic anhydride to convert the 3-aminoacetophenone into 3-acetamido-acetophenone; reacting the obtained 3-acetamido-acetophenone with powdered potassium hydrate first and then with iodomethane to convert the 3-acetamido-acetophenone into N-methyl-N-(3-acetyl-phenyl)-acetamide; reacting the obtained N-methyl-N-(3-acetyl-phenyl)-acetamide with ethyl formate under the action of sodium ethylate to obtain an alkali metal 3-(N-methyl-N-acetyl)amino-beta-ketone-phenyl-propionaldehyde enol salt; and synthesizing the indiplon by condensing the obtained alkali metal 3-(N-methyl-N-acetyl) amino-beta-ketone-phenyl-propionaldehyde enol salt and (3-amino-1H-pyrazole-4-yl)-2-thiophene ketone. The method has the advantages of low cost, mild reaction condition, safety and environmental protection, and easy industrialized production, and the obtained indiplon can be used as hypnagogue for treating anhypnosis.
Owner:ARMY MEDICAL UNIV
Genetic markers for skatole metabolism
InactiveUS7202035B2High activityReducing boar taintSugar derivativesMicrobiological testing/measurementAldehyde oxidaseM-aminoacetophenone
Novel metabolites and enzymes involved in skatole metabolism are disclosed. The novel metabolites are 3-OH-3-methylindolenine (HMI); 3-methyloxindole (3MOI); indole-3-carbinol (I-3C); and 2-aminoacetophenone (2-AM). Measuring levels of these metabolites in a pig may be useful in identifying the pig's ability to metabolize skatole and its susceptibility to boar taint. The novel enzymes involved in skatole metabolism are aldehyde oxidase and CYP2A6. Enhancing the activity of these enzymes may be useful in enhancing skatole metabolism and reducing boar taint. The identification of the enzyme also allows the development of screening assays for substances that interact with these enzymes and skatole metabolism or for genetic screening to identify pigs on the basis of their skatole metabolism. Pigs having high levels of these enzymes may be selected and bred to produce pigs that have a lower incidence of boar taint.
Owner:GUELPH UNIV OF
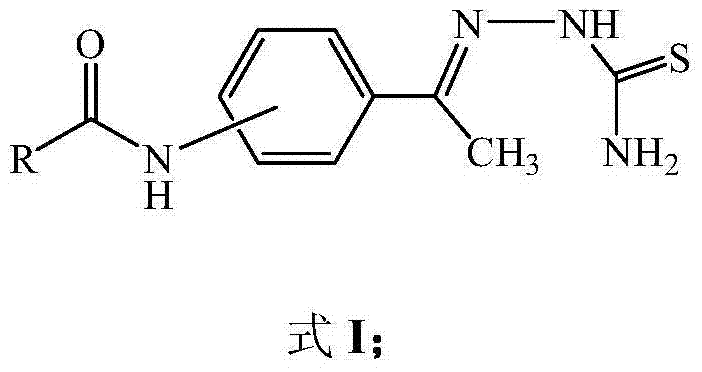
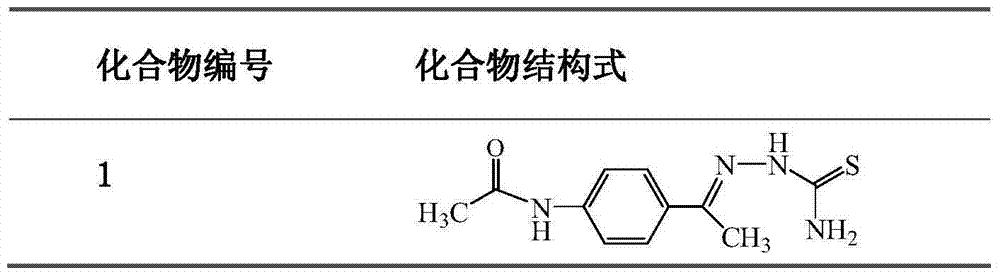
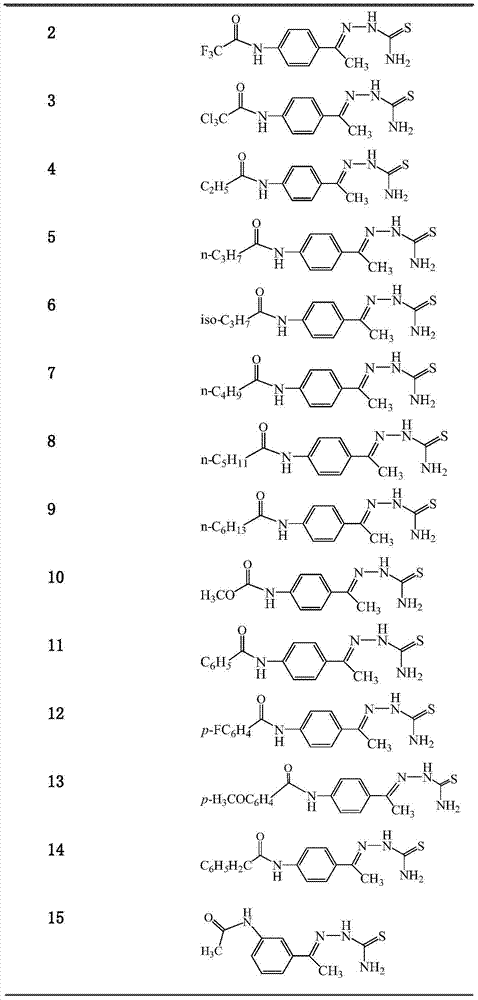
Aminoacetophenone thiosemicarbazone derivative and application thereof
ActiveCN104326957ANovel structureEasy to makeNervous disorderOrganic chemistryM-aminoacetophenoneAryl
The invention discloses an aminoacetophenone thiosemicarbazone derivative shown as a formula I and application thereof. In the formula I, an amino group connected with R is at 3 position or 4 position of the phenyl ring, and R is selected from C1-C6 alky or halogenated alkyl or alkoxy, aryl or substituted aryl. The related compounds are novel in structure and simple to prepare, are strong in inhibition activity on tyrosinase and have extremely obvious application prospect.
Owner:INST OF ANALYSIS GUANGDONG ACAD OF SCI (CHINA NAT ANALYTICAL
Compound, active energy ray curable composition, cured article thereof, printing ink, and inkjet recording ink
InactiveCN106414398APromote migrationImprove curing effectOrganic chemistryDuplicating/marking methodsM-aminoacetophenoneHydrogen atom
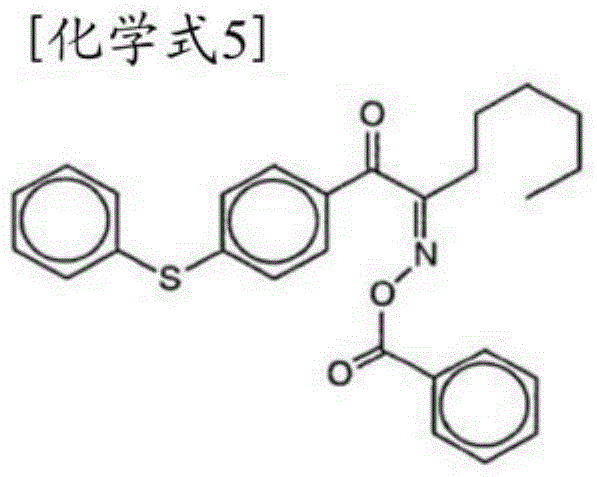
The present invention improves curability and reduces post-curing migration in a photopolymerization initiator. As a photopolymerization initiator, the present invention uses a compound obtained as a result of a Michael addition reaction between an alpha-aminoacetophenone skeleton-containing compound (I) represented by general formula (1), and a reactive compound (II) which functions as a Michael acceptor. (In formula (1), R1 represents an aliphatic group or the like, R2-R3 each independently represent an aliphatic group or the like, R4-R7 each independently represent a hydrogen atom or the like, X1 represents a single bond or a C1-6 alkylene group, X2 represents a carbonyl group or the like, and Y1 and Y2 represent a group represented by general formula (2) or the like. Meanwhile, when Y1 and Y2 both have a structure represented by general formula (2), the X5 in one or both is -NH-.) (In general formula (2), X3 and X4 each independently represent a C2-6 straight-chain or branched alkylene group or the like, and X5 represents a single bond, -O-, or -NH-).
Owner:DIC CORP
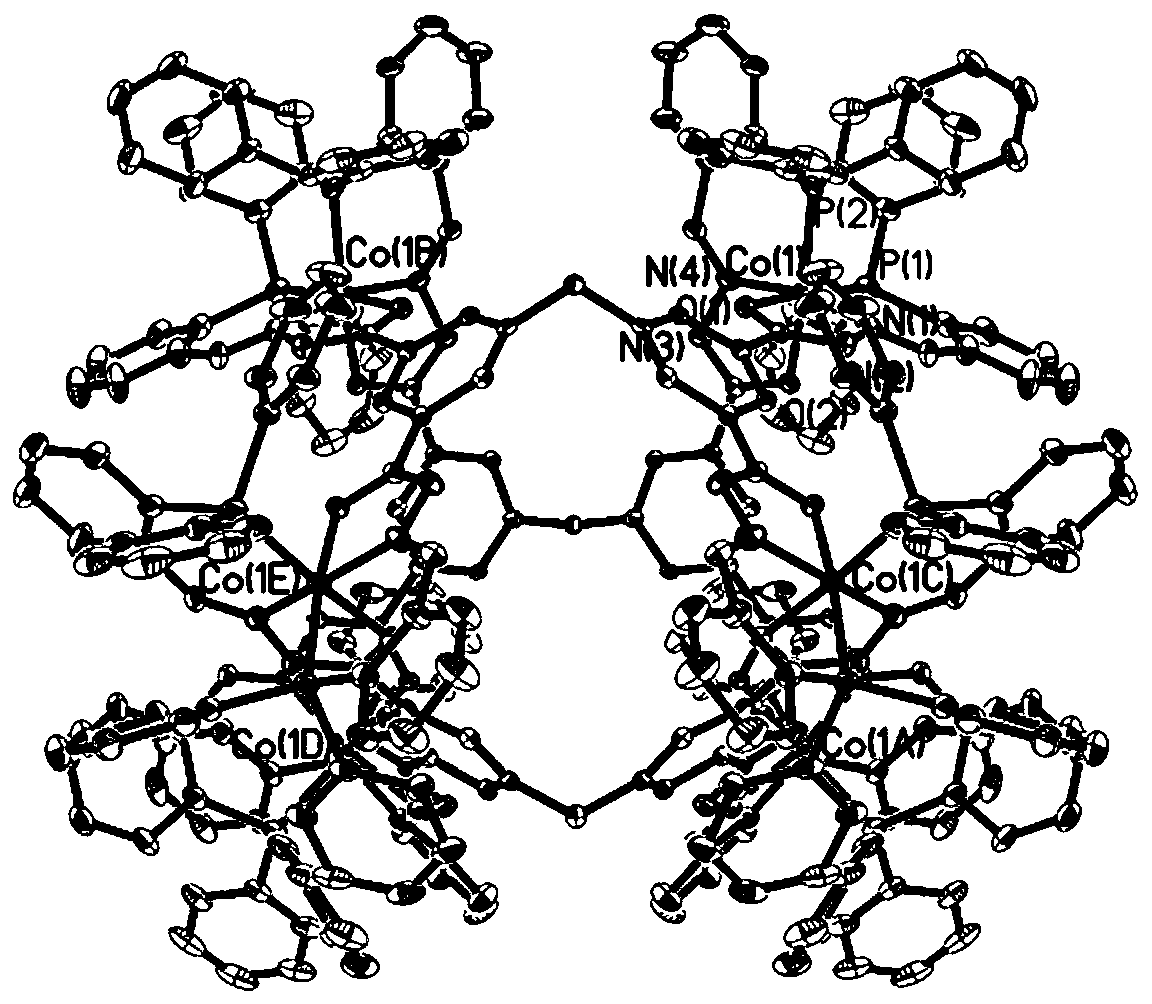
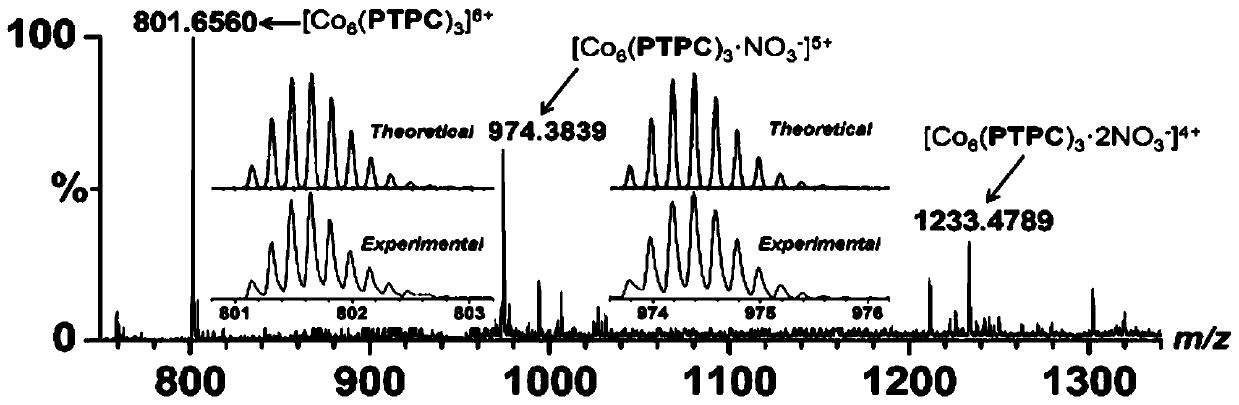
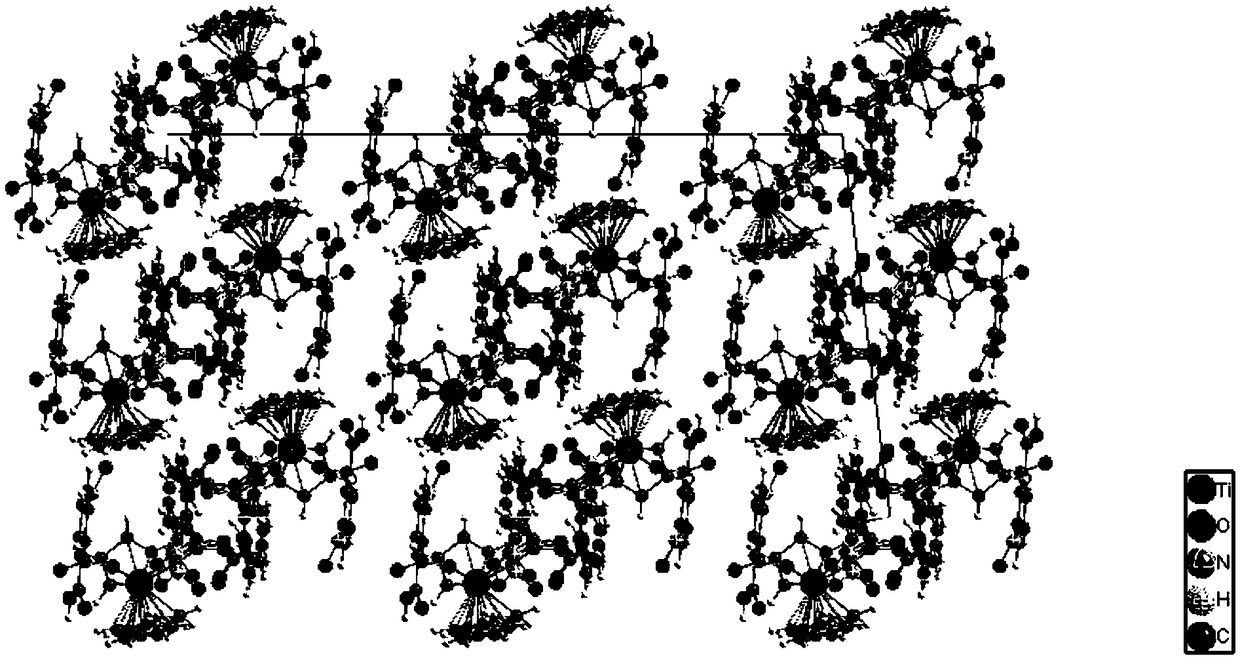
Preparation method and applications of metal organic triangular prism compound for preparing amine aromatic hydrocarbons through catalytic reduction of nitro aromatic hydrocarbons
ActiveCN110467636AChemically stableEasy to put inOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsM-aminoacetophenoneTetrafluoroborate
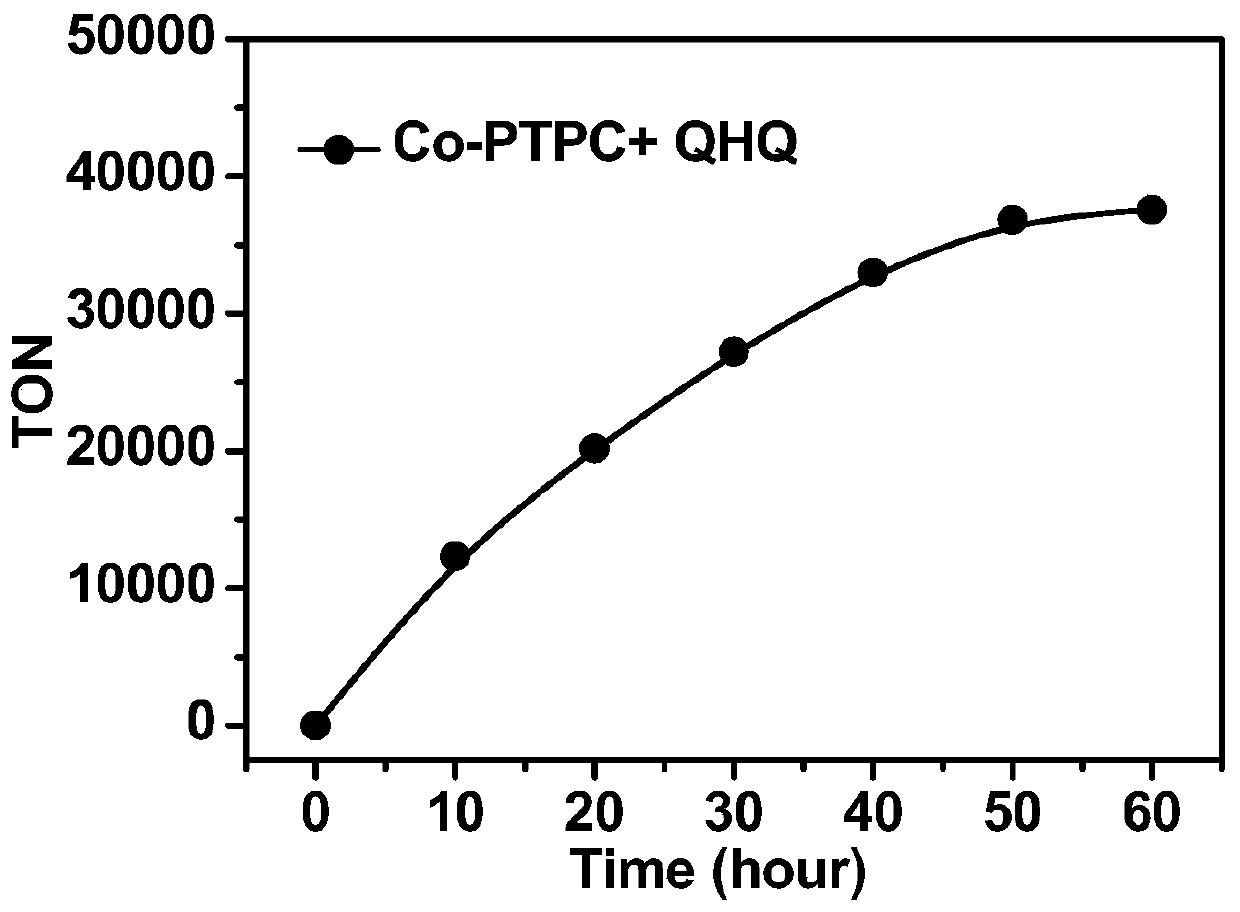
The invention belongs to the technical field of fine chemical industry, and relates to a preparation method and applications of a metal organic triangular prism compound for preparing amine aromatic hydrocarbons through catalytic reduction of nitro aromatic hydrocarbons. According to the preparation method, the metal organic triangular prism compound is prepared by using the Co<2+> in a transitionmetal salt as a node and using L as a ligand through a reaction; the synthesis route is Co<2+>+L [arrow] Co-L; and the ligand L is selected from H4PTPC, and the transition metal salt is one selectedfrom cobalt nitrate, cobalt perchlorate, cobalt tetrafluoroborate and cobalt trifluoromethanesulfonate. According to the present invention, the preparation method has characteristics of inexpensive raw materials and high yield, and the prepared metal organic triangular prism compound has stable chemical properties, and is easily used in practical application; and with the application of the prepared metal organic triangular prism compound as the compound Co-PTPC in the reduction of m-nitroacetophenone into m-aminoacetophenone and the reduction of p-nitroacetophenone into p-aminoacetophenone, the TON can be up to 36000, and the selectivity is greater than 99%.
Owner:DALIAN UNIV OF TECH
Preparation technology of high purity 3-hydroxyacetophenone
ActiveCN102249884AReduce usageSimple processOrganic compound preparationCarbonyl compound preparationM-aminoacetophenoneCharging order
A preparation technology of high purity 3-hydroxyacetophenone comprises the following steps of: adding 38 weight portions of 3-nitroacetophenone and 37 weight portions of water into a reaction vessel, adding an iron powder to react for 10 hours, adding methanol with stirring, centrifuging a precipitate for removing water to obtain 3-aminoacephenone, pouring the 3-aminoacephenone into a diazonium kettle; adding sulfuric acid, slowly adding a sodium nitrite aqueous solution into the diazonium kettle to produce 3-diazo sulfate acetophenone, adding 3-diazo sulfate acetophenone into a hydrolysis kettle, and finally drying to obtain the pure product 3-hydroxyacetophenone. The invention has advantages of simple technology, high yield of the product and high purity. By reasonable design of the reaction steps and adjustment of charging order, the application amount of strong acid is greatly reduced; in addition, by the addition of a certain amount of methanol in the reaction, the process of the reaction can be accelerated and the synthesis quality can be raised. According to statistics, the overall yield of the technology can reach over 92%; compared with other production technologies, the technology provided by the invention enables the product cost to decrease by more than 40%, and is environmentally friendly.
Owner:XIANTAO XIANSHENG FINE CHEM
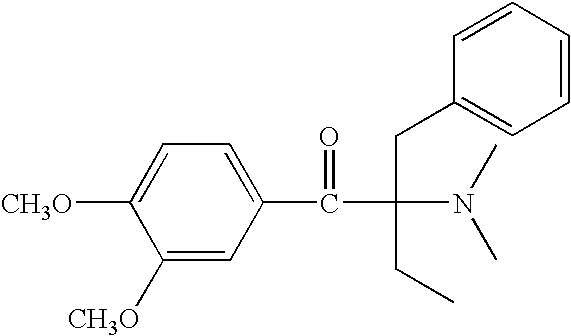
1-(4-morpholinylphenyl)-1-butanone preparation method
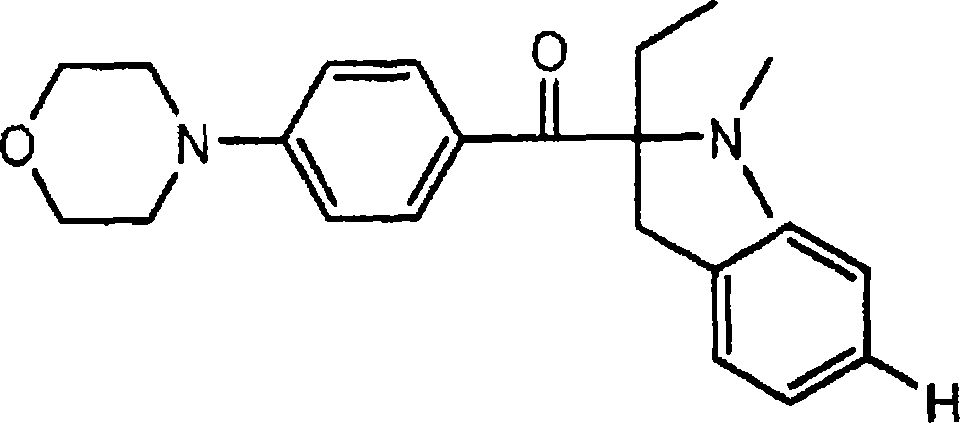
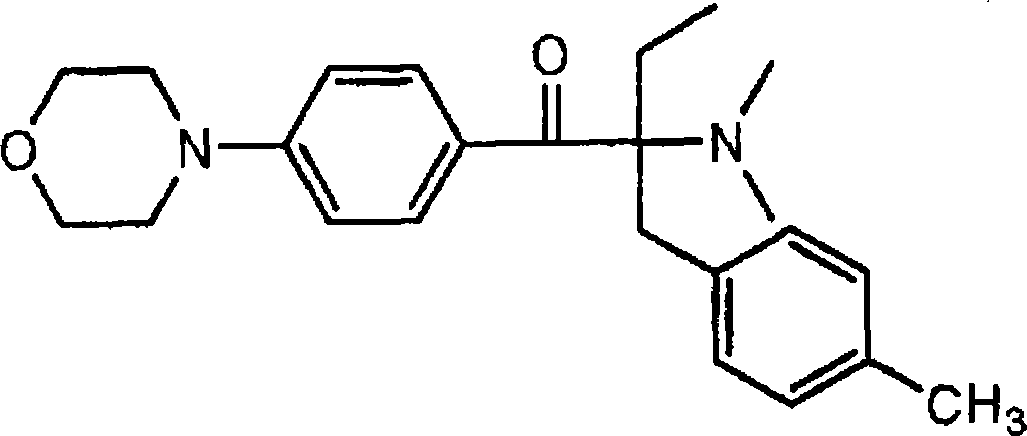
The invention relates to an alpha-aminoacetophenone photoinitiator intermediate preparation method, particularly to a preparation method of an intermediate of photoinitiators 2-benzyl-2-dimethylamino-1-(4-morpholinylphenyl)butanone and 2-(4-methylbenzyl)-2-dimethylamino-1-(4-morpholinylphenyl)butanone. According to the present invention, the disadvantages in the prior art are overcome, and the alpha-aminoacetophenone photoinitiator intermediate preparation method is provided, wherein the method does not require high-temperature and high-pressure feeding, has simple post-treatment, has good product appearance, and is suitable for industrial production.
Owner:TIANJIN JIURI NEW MATERIALS CO LTD
Quinoline derivative efficient catalytic synthesis method
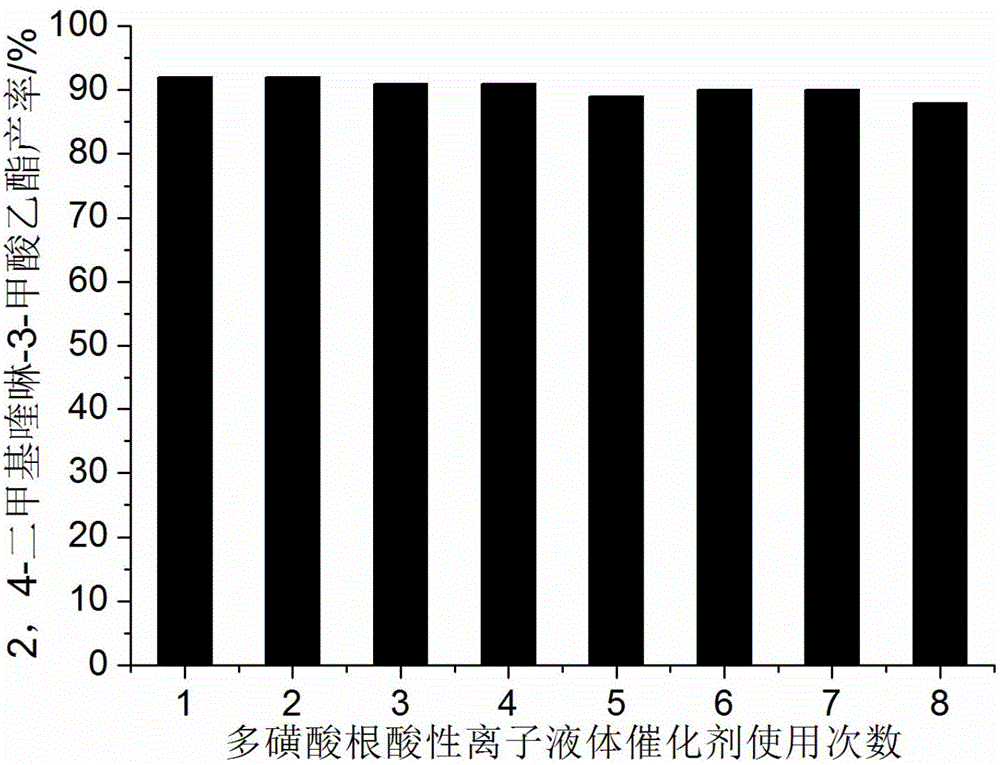
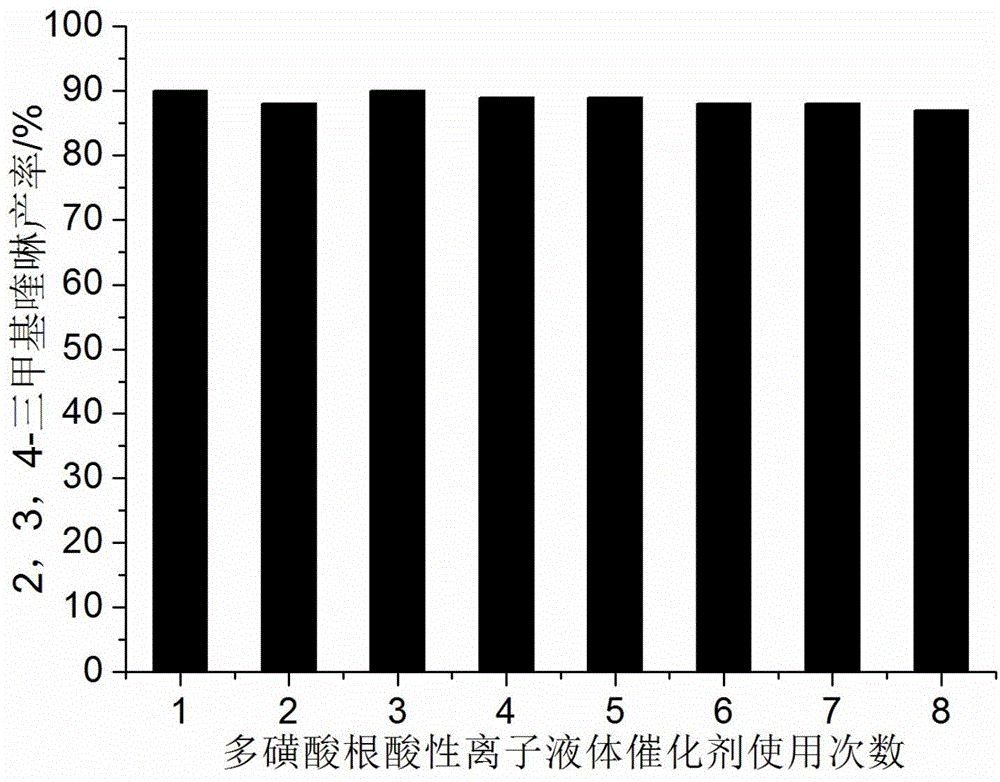
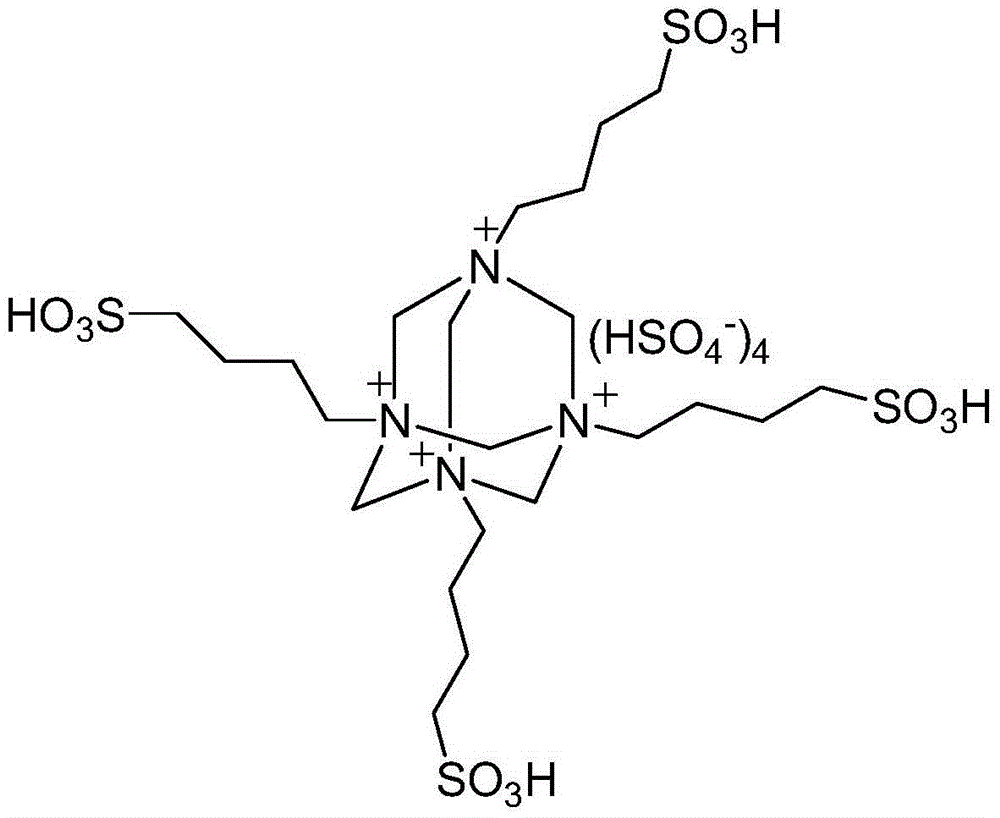
ActiveCN104311484AHigh acid densityHigh catalytic activityOrganic chemistryM-aminoacetophenoneSynthesis methods
The invention discloses a quinoline derivative efficient catalytic synthesis method belonging to the technical field of organic synthesis. The molar ratio of an active alpha-methyl or methylene carbonyl compound and 2-amino acetophenone is 1: 1, the molar amount of a multi sulfonic acid r adical acidic ionic liquid catalyst is 7-10% of the use amount of the 2-amino acetophenone, the reaction solvent 75% ethanol aqueous solution volume (ml) is 3-5 times of the molar amount (mmol) of the 2-amino acetophenone, the reflux reaction time is 5-25min, a filter residue is obtained by cooling to room temperature after the reaction and filtering, and the obtained filter residue is dried under vacuum to obtain a pure quinoline derivative. Compared with a synthesis method using other acidic ionic liquid as a catalyst, the catalyst has high catalytic activity, less use amount and less loss quantity during the circulation use, and the whole synthetic process has the advantages of being simple, convenient, economic and the like, and is convenient for industrialized mass production.
Owner:南京苏亦欣医药科技有限公司
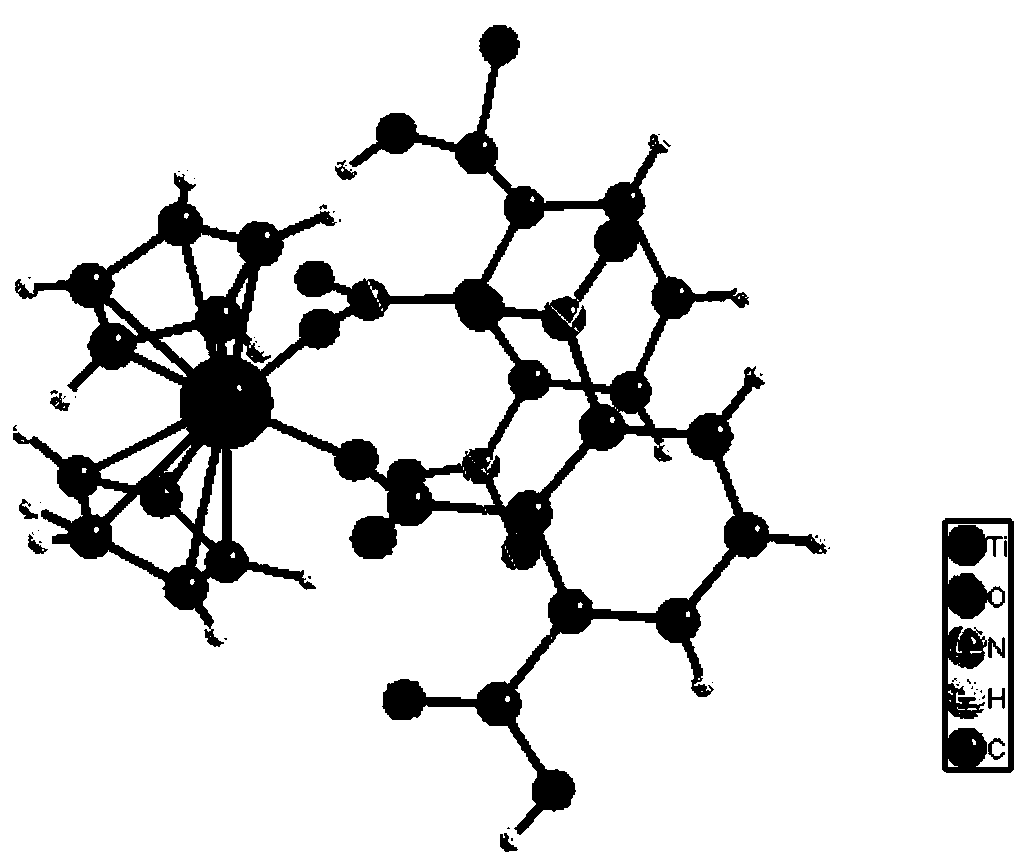
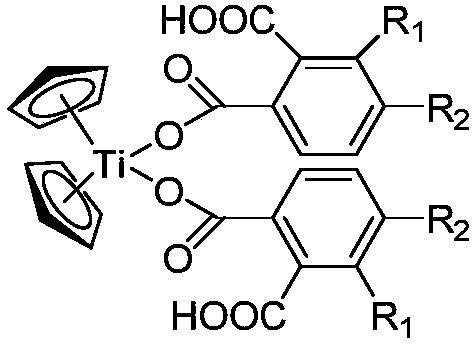
Titanocene complex with oxygen-containing carboxylic acid as ligand as well as preparation method and application of titanocene complex
InactiveCN108659056APreparation raw materials are cheap and easy to obtainEasy to prepare ingredientsOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsM-aminoacetophenoneSynthesis methods
The invention discloses a titanocene complex with oxygen-containing carboxylic acid as well as a preparation method and an application of the titanocene complex. The structural formula of the complexis shown in the description. The titanocene complex is prepared from oxygen-containing carboxylic acid as a ligand, titanocene dichloride as Lewis acid and 5-sulfosalicylic acid as a transitional ligand. The complex is stable to air, the raw materials used in the synthesis method are low in cost and easy to obtain, synthesis steps are simple and easy, post-treatment of the product is simple, conditions are mild, and the yield of the target product is high. The complex is used for catalyzing reaction of 2-aminoacetophenone and aromatic aldehyde for synthesis of 2-aryl-2,3-dihydroquinolinone, and better yield is obtained.
Owner:SHAANXI NORMAL UNIV +1
Method for removing ethanol, isopropanol and octyl alcohol from amino resin workshop wastewater
InactiveCN105084495AStrong complexing abilityFast precipitationWater contaminantsNature of treatment waterOctanolAcetophenone
The invention relates to a method for removing ethanol, isopropanol and octyl alcohol from amino resin workshop wastewater. The components adopted by the method comprises 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane, 1,8-dihydroxy-3-methoxy-6-methylanthraquinone, S(-)-2-amino-6-n-propyle-4,5,6,7-tetrahydrobenzothiazole dihydrochloride, 4-octylphenol ethoxylate, 3',4'-dihydoxy-2-(methylamino)acetophenone hydrochloride, 4-acetoxy-3-methoxy-(2-propenyl) benzene, 4-methoxybenzyl acetate, 6,6,10-trimethyl bicyclo-3,1,1-hept-2-ene, and 2-[[1-(3-acetylthio-2-methylpropionyl)pyrrolidine-2-formyl]amino]-3-phenylpropionic acid. The components adopted by the method have strong complexing capability with target substances, high speed of forming complex precipitates, and high removal rate up to 99.9%.
Owner:李海兰
Stable isotope labeling Clenproperol compound and synthesis method thereof
InactiveCN105968021AAtom utilization is highSimple process routeOrganic compound preparationOrganic chemistry methodsM-aminoacetophenoneStable Isotope Labeling
The invention relates to a stable isotope labeling Clenproperol compound and a synthesis method thereof. The method comprises the following steps of (1) using stable isotope labeling 2,6-dichloroaniline as raw materials to generate stable isotope labeling 3,5-dichloro-4-aminoacetophenone through Friedel-Crafts acyl reaction; (2) performing bromination on the stable isotope labeling 3,5-dichloro-4-aminoacetophenone to obtain stable isotope labeling 3,5-dichloro-4-amino-alpha-bromo acetophenone; (3) enabling the stable isotope labeling 3,5-dichloro-4-amino-alpha-bromo acetophenone and isopropylamine to take a reaction for obtaining stable isotope labeling 1-(4-amino-3,5-dichlone)-2-isopropyl amino ethyl ketone; (4) enabling the stable isotope labeling 1-(4-amino-3,5-dichlone)-2-isopropyl amino ethyl ketone and a reducing agent to take a reaction to generate stable isotope labeling Clenproperol. The stable isotope labeling Clenproperol compound and the synthesis method have the advantages that the process route is simple; the synthesis is easy; the stable isotope atom utilization rate is high; the obtained product can be easily separated and purified; the chemical purity and the fractional isotopic abudance are 99 percent or higher; the trace detection requirements in the food safety field can be sufficiently met.
Owner:SHANGHAI RES INST OF CHEM IND
Method for synthesizing 2,3í»-dichloroacetophenone
InactiveCN101333157AOrganic compound preparationCarbonyl compound preparationM-aminoacetophenoneSandmeyer reaction
The invention relates to a synthesis method for 2,3'-dichloroacetophenone (alpha-chloro-acetophenone inter-chlorophenyl) and is characterized in that the method takes inter-amino-acetophenone as raw material to generate between inter-acetophenone diazo-hydrochloride through diazotization reaction, or generate inter-p-acetophenone through Sandmeyer reaction, or generate 2,3'-dichloroacetophenone through alpha-chloro-reaction; the synthetic way is simple and the conditions are mild, with a total yield rate more than 80%.
Owner:徐州诺特化工有限公司
Photosensitive combination and photosensitive transprinting material using same, display device shading film and manufacturing method, black matrix, substrate having shading film and display device
InactiveCN101071264AHigh sensitivityPhotomechanical apparatusNon-linear opticsComing outM-aminoacetophenone
The present invention provides a photosensitive compound and a photosensitive transfer-printing material using the photosensitive compound, and the lightproof film which is used for the display device and the making method, black matrix, cardinal plate with lightproof film and the display device, the photosensitive compound will not come out the agglomeration of the metal particle, and the sensitivity is good when it is used as photosensitive material in a long time. The photosensitive transfer-printing material of the invention is characterized in that it at least includes: (A) metal particles or particle comprising metal, (B) at least one photopolymerization initiator choosing from the free aminoacetophenone, acyl phosphine oxide and the group composed of oxime-ester, (C) resin and at least one kind of its precursors.
Owner:FUJIFILM CORP
Preparation method for special syrup for rainbow candy
The invention provides a preparation method for special syrup for a rainbow candy. The preparation method comprises the following steps: mixing a corn starch and water to form a corn slurry, adding astarch milk B, and preparing the special syrup for the rainbow candy through the processes of liquifying, saccharifying, mixing, filtering, decolorizing, hybridizing, adsorbing through polymerizing foreign compounds with special resin, and evaporating. The glucose content in the produced special syrup for the rainbow candy is 42.0-46.0%, the maltose content is 25.0-32.0%, the DE value is 63-70%, and the chromaticity is less than or equal to 10. Through the polymerized adsorption, the hydroxymethyl furfural of a product is controlled to be lower than or equal to 75 ppm, the furfural is controlled to be lower than or equal to 2.0 ppm, the acetaldehyde is controlled to be lower than or equal to 80 ppb, the diaminoacetophenone (2AP) is controlled to be lower than or equal to 0.5 ppb, the isovaleraldehyde is controlled to be lower than or equal to 5.0 ppb, the influence of an odorous compound on the sensory threshold is reduced, and the adverse effect on the flavor of the product is avoided, so that the taste and the flavor of the rainbow candy are guaranteed to be pure.
Owner:河南飞天生物科技股份有限公司
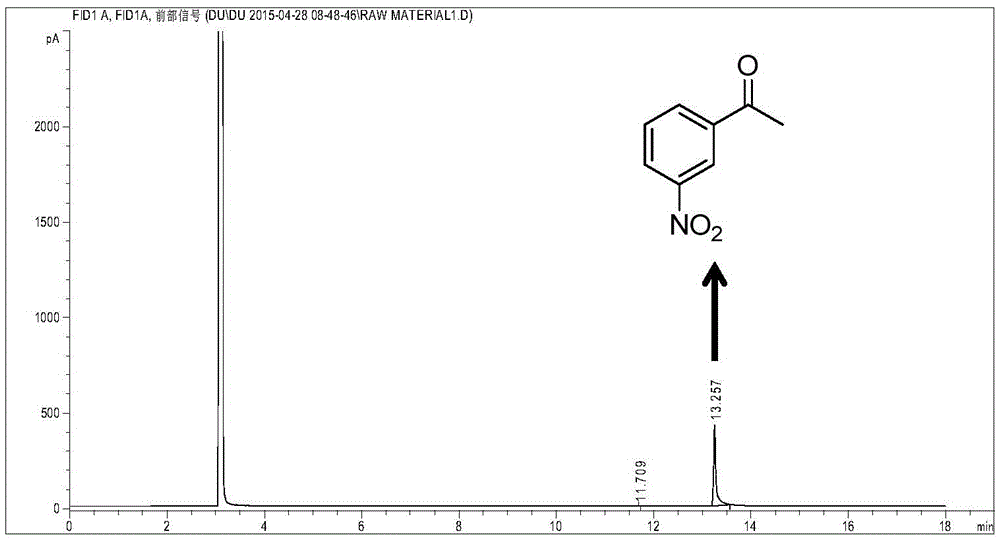
Method for catalytic reduction of m-nitroacetophenone for preparation of m-aminoacetophenone
InactiveCN105566131AHigh yieldIncrease the number of applicationsOrganic chemistryOrganic compound preparationM-aminoacetophenoneBismuth compound
The present invention provides a method for catalytic reduction of m-nitroacetophenone for preparation of m-aminoacetophenone, according to the method, hydrogen is used as a reducing agent, bismuth-compound-supported Pt is used as a catalyst, and the m-nitroacetophenone is highly-selectively reduced into the m-aminoacetophenone by a batch-type one-step reaction. The method reaction temperature is 30-120 DEG C, the reaction time is 1-20 hours, and the hydrogen partial pressure is 0.05-2.0MPa. The catalyst system can be used for efficiently catalytic hydrogenation of the m-nitroacetophenone, the m-aminoacetophenone yield is high; the hydrogen is used as the reducing agent, a main by-product is water, the method is green and environmentally-friendly; reaction conditions are mild, hydrogenation process equipment requirements are low, operation is simple; product separation and purification are simple, and the number of reusing times of the catalyst is high.
Owner:DALIAN UNIV OF TECH
Photosensitive Resin Composition And Spacer Preprared From The Same
InactiveCN104035278AImprove photoresponse performanceHigh sensitivityPhotomechanical exposure apparatusPhotosensitive materials for photomechanical apparatusM-aminoacetophenoneHydrogen
The present invention relates to a photosensitive resin composition and a spacer prepared from the same. In specific, the photosensitive resin composition includes an alkali-soluble resin, a photocurable monomer, a photopolymerization initiator, an amino acetophenone-based or amino benzaldehyde acetophenone-based hydrogen donor, and a solvent, thereby improving photoreactivity by activating alkyl radicals generated from the photopolymerization initiator and providing improved sensitivity by maximizing light efficiency. Accordingly, the sensitivity of the photosensitive resin composition is largely improved.
Owner:DONGWOO FINE CHEM CO LTD
Chalcone derivatives, pharmaceutically acceptable salt, method for preparation and uses thereof
InactiveUS7851654B2Easy to useStrong enzyme inhibitory activityBiocideCosmetic preparationsM-aminoacetophenoneDisease
Owner:JCN FARM CO LTD
Synthesizing process of 3-methyl-1 H-indazole
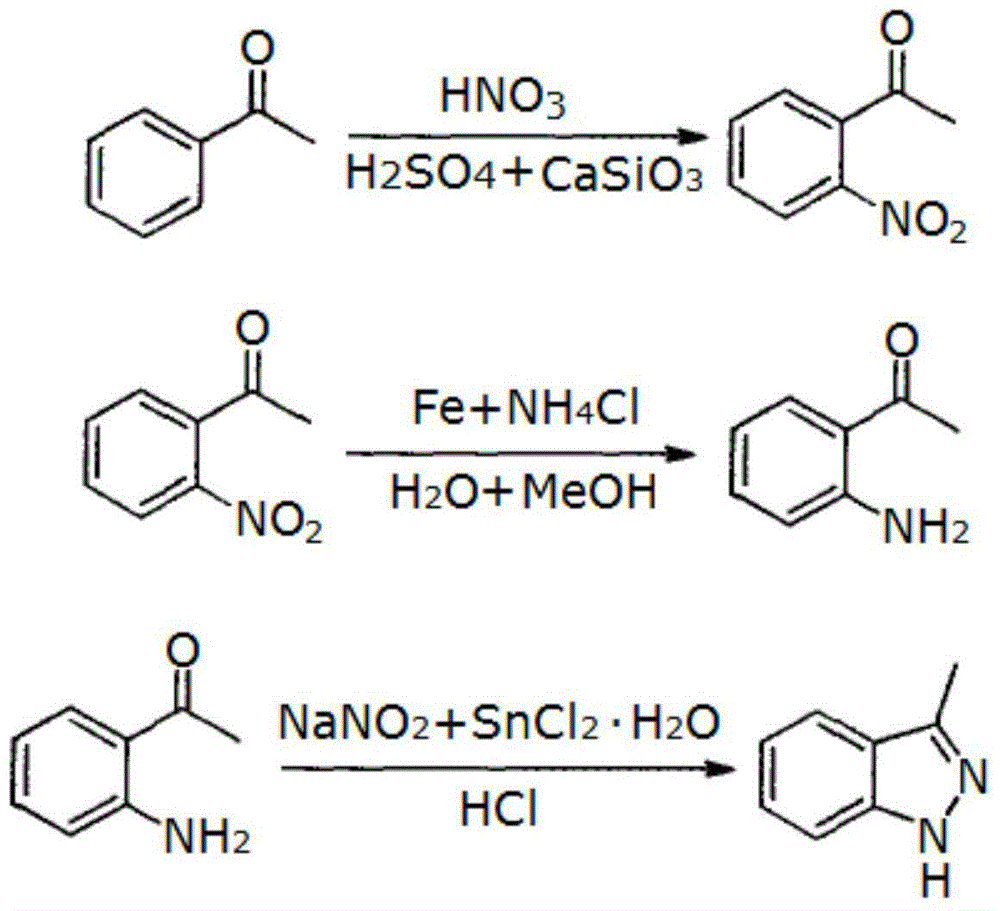
ActiveCN105198813AHigh yieldRaw materials are easy to getOrganic chemistryCalcium silicateM-aminoacetophenone
The invention relates to a synthesizing process of 3-methyl-1 H-indazole. The synthesizing process comprises the following steps: acetophenone is dropped into a sulfuric acid and nitric acid mixture solution, then calcium metasilicate powder is added, the mixture is kept to be stirred at lower temperature and passes through the night, the mixture is added into ice water and filtered, and 2-nitroacetophenone is obtained; the 2-nitroacetophenone, iron powder and ammonium chloride are synthesized into white solid 2-aminoacetophenone; the 2-aminoacetophenone is added to hydrochloric acid, then an NaNO2 aqueous solution is added, the mixture is stirred, then a hydrochloric acid solution of SnCl2.H2O is added, the mixture is stirred, the mixture is added to ice water and filtered, and filtrate is adjusted to alkalescence, filtered and dried so that the 3-methyl-1 H-indazole is obtained. According to the synthesizing process of the 3-methyl-1 H-indazole, calcium silicate is taken as a catalyst, the yield of the 2-nitroacetophenone is increased remarkably, the raw material of calcium silicate is easy to obtain, the cost is low, the operation and the use are simple, later synthesis of 2-aminoacetophenone and the 3-methyl-1 H-indazole is further facilitated, and the synthesizing process is suitable for industrial large-scale synthesis of 3-methyl-1 H-indazole.
Owner:上海泰坦科技股份有限公司
Preparation method of o-aminoacetophenone
PendingCN110938008ASufficient supplyLow priceOrganic chemistryOrganic compound preparationM-aminoacetophenonePtru catalyst
The invention discloses a preparation method of o-aminoacetophenone. The preparation method comprises the following steps: (1) mixing o-nitroethylbenzene, an oxidant, a cocatalyst and an organic solvent, heating the obtained mixed solution to a certain temperature for a reaction, and subjecting the reacted solution to cooling, quenching and extracting to obtain o-nitroacetophenone; and (2) addinga polar solvent and a metal catalyst into the obtained o-nitroacetophenone, carrying out a hydrogenation reduction reaction to obtain a crude o-aminoacetophenone product, and filtering and rectifyingthe crude o-aminoacetophenone product to obtain a refined o-aminoacetophenone product. According to the invention, used raw materials are low in price and easy to obtain, and steps are simple and easyto operate. The o-aminoacetophenone obtained by the method disclosed by the invention is high in purity and high in yield.
Owner:NANJING JIEYUN PHARMA TECH CO LTD
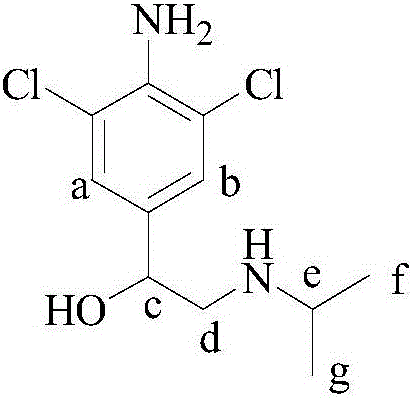
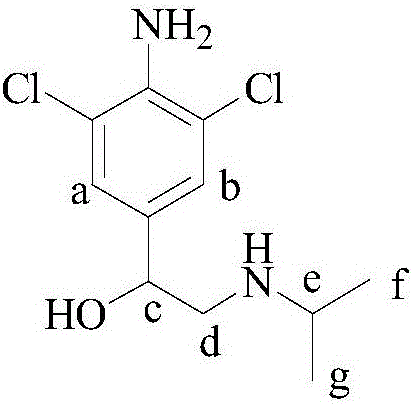
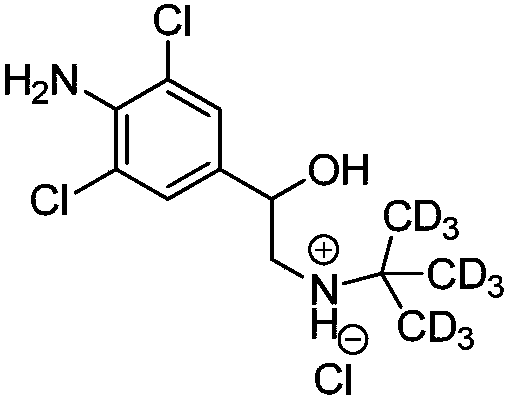
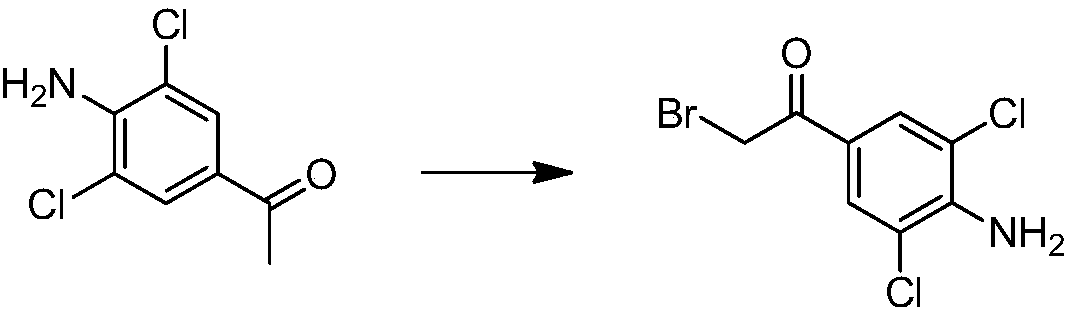
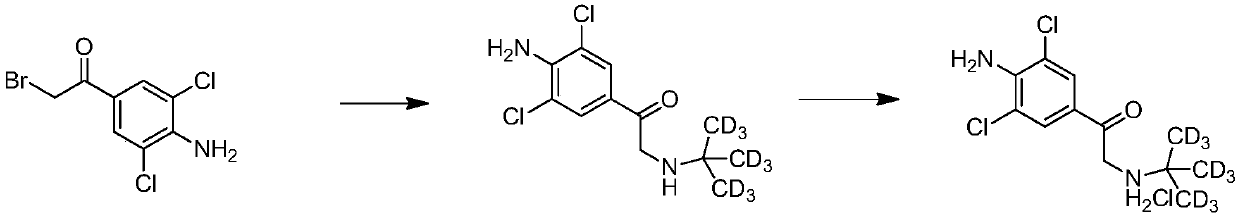
Synthetic method of deuterium-labeled D9-clenbuterol hydrochloride
ActiveCN109096126AEasy to operateSimple processing methodOrganic compound preparationOrganic chemistry methodsM-aminoacetophenoneTert-Butylamine
The invention belongs to the field of food safety and standard substance synthesis, and discloses a synthetic method of deuterium-labeled D9-clenbuterol hydrochloride. According to the method, 4-amino-3,5-dichloroacetophenone is taken as a raw material and subjected to three-step reaction including bromination, D9 tert-butyl amination and reduction, and deuterium-labeled D9-clenbuterol hydrochloride is generated. The method is simple to operate, reaction conditions are mild (most reactions are performed at room temperature), reaction routes are reasonable, the process is advanced, the processing method is simple, and a product is easy to purify. Meanwhile, the total conversion rate of D9-tert-butylamine as the core raw material in the synthesis process of D9-clenbuterol hydrochloride is increased to 40% from 4.2%, yield is increased to 64%, and the method has good economic value and social value.
Owner:东莞暨南大学研究院 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com