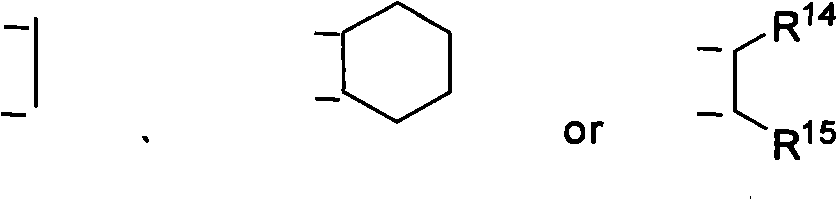
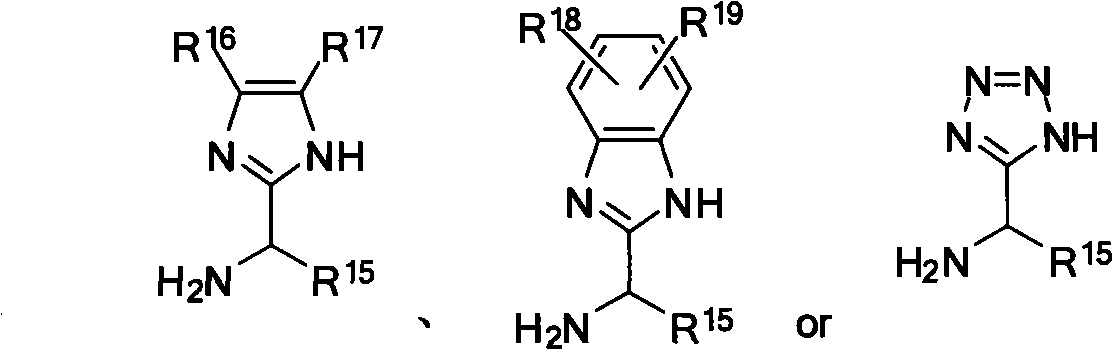
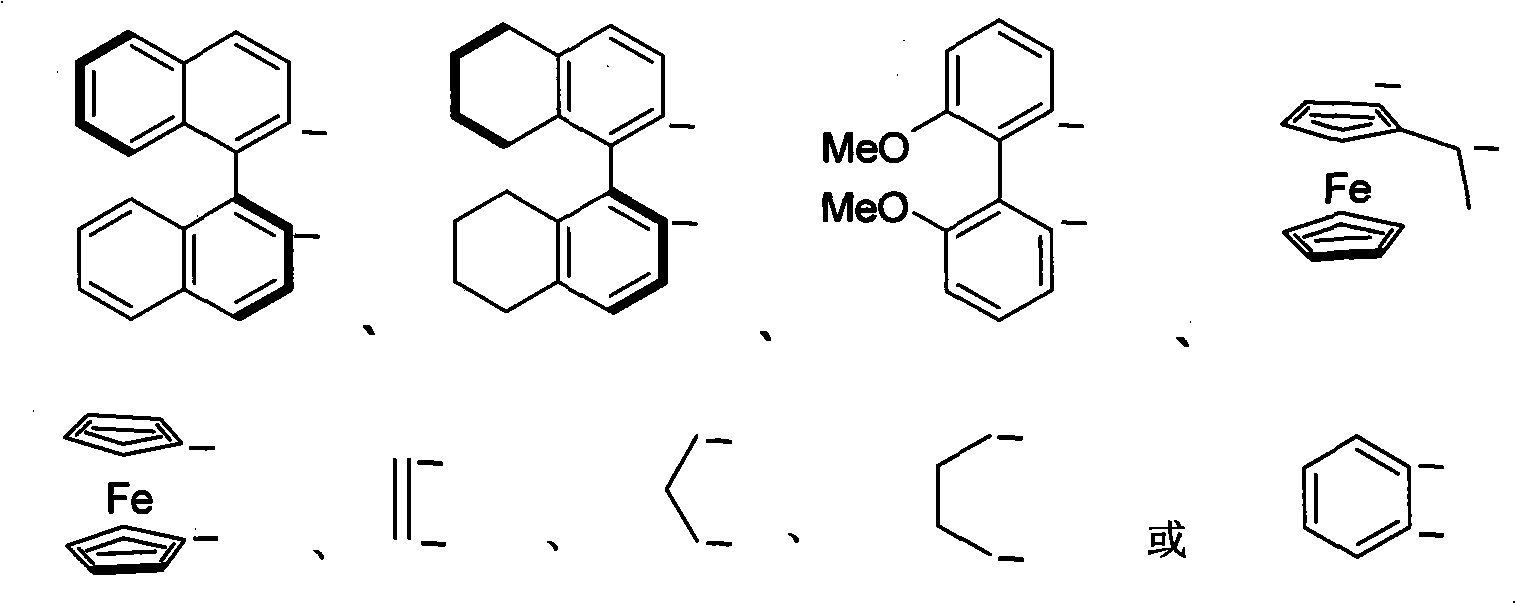
Nitrogen-containing ligand transient metal complex compound , synthetic method and use thereof
A technology of transition metals and synthesis methods, applied in the field of nitrogen-containing ligand transition metal complexes and their synthesis, to achieve the effect of simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0037] Example 2: Catalyst 9
[0038]
[0039] Using Method 1, the yield: 74%.
[0040] 31 P NMR (121MHz, CDCl 3 ): δ42.80, 44.62ppm.
[0041] Method Two
[0042]
Embodiment 3
[0043] Embodiment 3: the preparation of catalyst 10
[0044] General method (method two): 16.4mg (0.026mmol) S-Binap and 6.6mg (0.053mmol) [RuCl 2 (C 6 h 6 )] 2 into the reaction tube. Add 2ml of anhydrous DMF, put it in an oil bath at 100°C, and stir for 30 minutes. After cooling down to 50°C and draining the solvent with an oil pump, add 4.3mg (0.026mmol) (S)-6 and 2mL anhydrous oxygen-free toluene, stir at room temperature for 1 hour, and then heat to reflux for 1 hour. When pumping under reduced pressure until about 0.5 mL of solvent remained, 5 mL of Hexane was added to settle, and 19 mg of yellow powder was obtained by elbow filtration. Yield: 71%.
[0045] 31 P NMR (121MHz, CDCl 3 ): δ49.58ppm (d, J=38.7Hz), 47.32ppm (d, J=38.7Hz); MS (MALDI) m / z: 884 [M-71] + .
Embodiment 4
[0046] Example 4: Catalyst 11
[0047]
[0048] Using method two, yield: 86%.
[0049] 31 P NMR (121MHz, CDCl 3 ): δ47.61ppm, 49.15ppm.
[0050] method three
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com