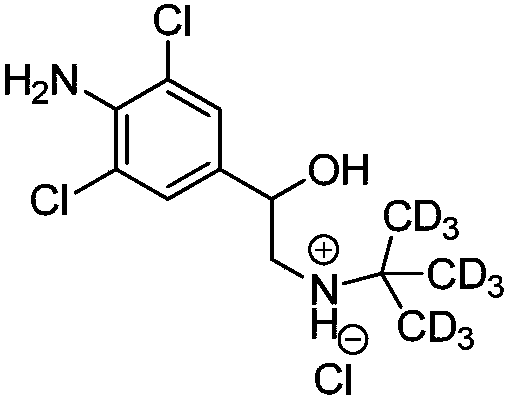
Synthetic method of deuterium-labeled D9-clenbuterol hydrochloride
A technology of clenbuterol hydrochloride and its synthetic method, which is applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds, can solve problems such as poor stability, and achieve advanced process selection, simple operation, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
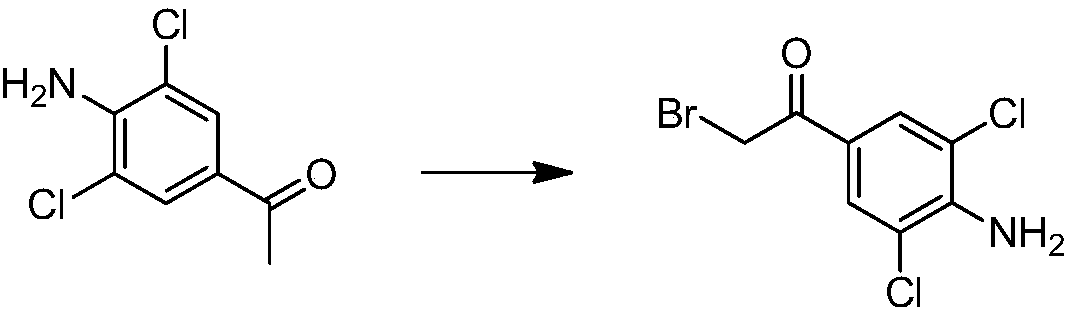
[0043]In a 250ml three-neck flask, add 4.8g of 3,5-dichloro-4-aminoacetophenone, 10.8g of copper bromide, 48ml of ethyl acetate and 48ml of chloroform, and reflux for 4 hours after the addition. Filter, wash the filter cake with ethyl acetate, combine the filtrates, wash twice with water, wash twice with saturated brine, dry over sodium sulfate, recrystallize with chloroform under reduced pressure, filter, and dry to obtain solid 3,5-dichloro-4-amino- 4.0 g of α-bromoacetophenone, the yield is about 62%.
[0044] The proton nuclear magnetic spectrum data of gained solid is as follows: 1 H NMR (CDCl 3 )δ7.85(s, 2H), 5.06 (s, 2H), 4.31(s, 2H). It shows that 3,5-dichloro-4-amino-α-bromoacetophenone was successfully synthesized in this example.
Embodiment 2
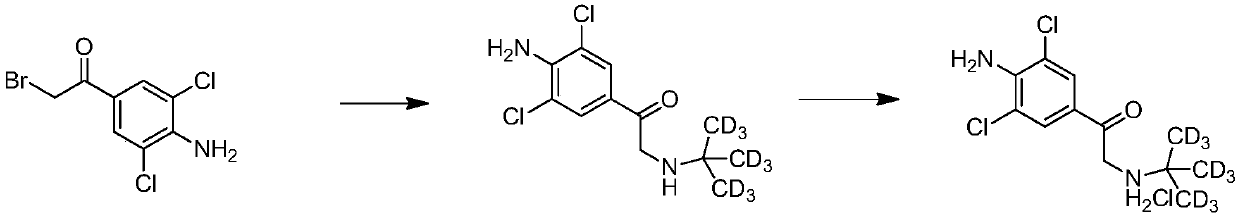
[0046] In a 50ml pressure bottle, add 2.8g of 3,5-dichloro-4-amino-α-bromoacetophenone, D 9 - tert-butylamine 1.2g, 45ml tetrahydrofuran, stirred and reacted at room temperature for 6 hours, and filtered to remove D generated during the reaction 9 - tert-butylamine hydrobromide, the filter cake was washed with 10ml THF, the filtrate was combined, about half of the THF was concentrated, and 4mol / L hydrogen chloride / ethanol solution was added to adjust the pH value of the solution to 4, filtered, and dried to obtain 4-amino-α- D. 9 - tert-butylamino-3,5-dichloroacetophenone hydrochloride 3.0g, yield 93%, D 9 - 63% conversion of tert-butylamine.
[0047] The proton nuclear magnetic spectrum data of gained solid is as follows: 1 H NMR (MeOD) δ8.00 (s, 2H), 4.59 (s, 2H), indicating that Example 2 successfully synthesized 4-amino-α-D 9 - tert-butylamino-3,5-dichloroacetophenone hydrochloride.
Embodiment 3
[0049] In a 25ml pressure bottle, add 1.0g of 3,5-dichloro-4-amino-α-bromoacetophenone, D 9 - 0.46g of tert-butylamine, 15ml of acetonitrile, stirred and reacted at room temperature for 6 hours, filtered, washed the filter cake with 5ml of acetonitrile, combined the filtrate, concentrated about half of the acetonitrile, added 4mol / L hydrogen chloride / ethanol solution until the solution was acidic (pH = 4), filtered and dried to obtain 4-amino-α-D 9 - tert-butylamino-3,5-dichloroacetophenone hydrochloride 1.1g, yield 96%, D 9 - The conversion of tert-butylamine is 61%. Wherein the proton nuclear magnetic spectrum data of drying gained solid is as follows: 1 H NMR (MeOD) δ8.00 (s, 2H), 4.59 (s, 2H), indicating that Example 3 also successfully synthesized 4-amino-α-D 9 - tert-butylamino-3,5-dichloroacetophenone hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com