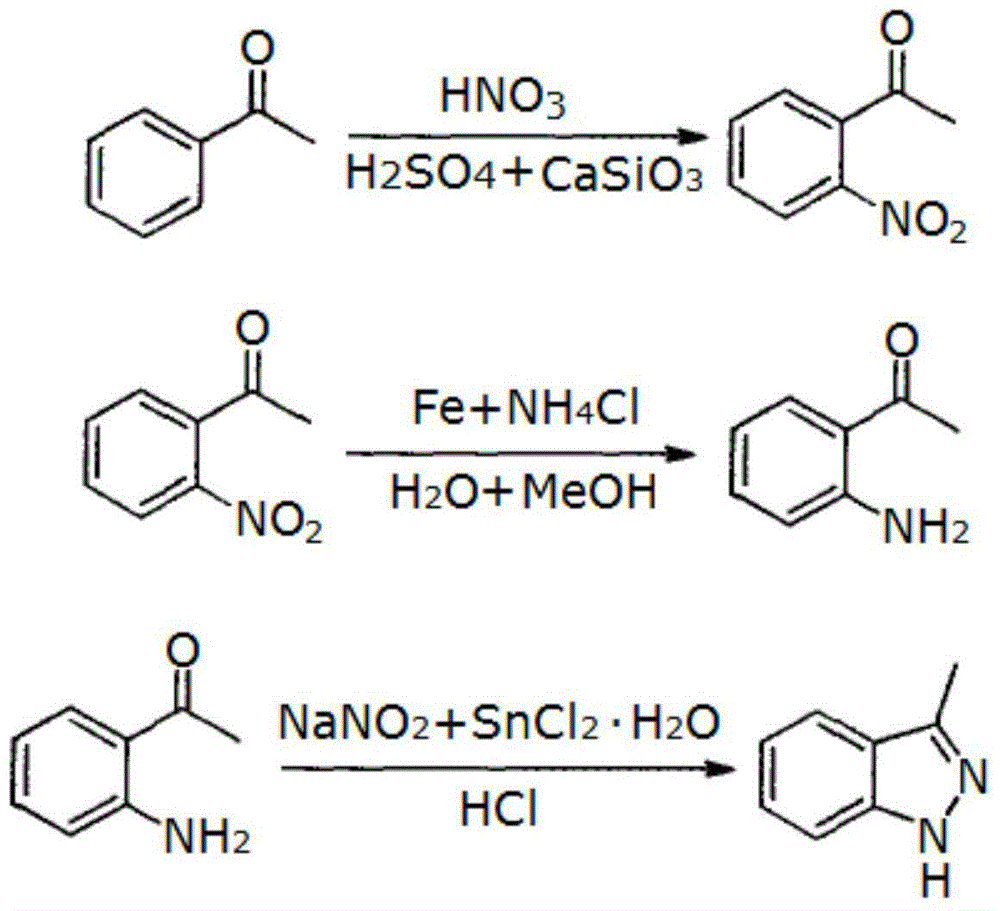
Synthesizing process of 3-methyl-1 H-indazole
A synthesis process, the technology of indazole, which is applied in the field of synthesis process of indazole derivatives, can solve the problems of low yield, unfavorable large-scale synthesis and production of 3-methyl-1H-indazole, etc., and achieve simple steps and low cost , the effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] This embodiment relates to a synthesis process of 3-methyl-1H-indazole, which consists of the following steps:
[0019] Step 1: Synthesis of 2-nitroacetophenone
[0020] Slowly drop 100g of acetophenone into 500ml of sulfuric acid and nitric acid mixture (the volume ratio of concentrated sulfuric acid and concentrated nitric acid is 1:7, the temperature is -15°C), and then add 14g of 90 mesh calcium silicate powder (acetophenone and silicic acid The weight ratio of calcium powder is 1:0.14), kept stirring overnight below -15°C, then added to ice water, filtered to obtain 134g of yellow solid 2-nitroacetophenone, yield 97%, purity 99.8%, MS:m / z=165(M + ), elemental analysis: C58.1% (theoretical value 58.2%), H4.2% (theoretical value 4.2%), O29.0% (theoretical value 29.1%), N8.4% (theoretical value 8.5%), by Molecular weight and elemental analysis confirmed that it was 2-nitroacetophenone;
[0021] Step 2: Synthesis of 2-aminoacetophenone
[0022] Dissolve 110 g of t...
Embodiment 2
[0026] This embodiment relates to a synthesis process of 3-methyl-1H-indazole, which consists of the following steps:
[0027] Step 1: Synthesis of 2-nitroacetophenone
[0028] Slowly drop 100g of acetophenone into 500ml of sulfuric acid and nitric acid mixture (volume ratio of concentrated sulfuric acid and concentrated nitric acid is 1:7, temperature -15°C), and then add 23g of 80 mesh calcium silicate powder (acetophenone and silicic acid The weight ratio of calcium powder is 1:0.23), kept stirring overnight below -15°C, then added to ice water, filtered to obtain 131g of yellow solid 2-nitroacetophenone, yield 95%, purity 99.6%, MS:m / z=165(M + ), elemental analysis: C58.0% (theoretical value 58.2%), H4.2% (theoretical value 4.2%), O29.2% (theoretical value 29.1%), N8.4% (theoretical value 8.5%), by Molecular weight and elemental analysis confirmed that it was 2-nitroacetophenone;
[0029] Step 2: Synthesis of 2-aminoacetophenone
[0030] Dissolve 110 g of the above-me...
Embodiment 3
[0034] This embodiment relates to a synthesis process of 3-methyl-1H-indazole, which consists of the following steps:
[0035] Step 1: Synthesis of 2-nitroacetophenone
[0036] Slowly drop 100g of acetophenone into 500ml of sulfuric acid and nitric acid mixture (volume ratio of concentrated sulfuric acid and concentrated nitric acid is 1:7, temperature -15°C), then add 6g of 100 mesh calcium silicate powder (acetophenone and the The weight ratio of calcium silicate powder is 1:0.06), kept below -15°C and stirred overnight, then added to ice water, filtered to obtain 132g of yellow solid 2-nitroacetophenone, yield 96%, purity 99.7%, MS :m / z=165(M +), elemental analysis: C58.2% (theoretical value 58.2%), H4.1% (theoretical value 4.2%), O29.0% (theoretical value 29.1%), N8.4% (theoretical value 8.5%), by Molecular weight and elemental analysis confirmed that it was 2-nitroacetophenone;
[0037] Step 2: Synthesis of 2-aminoacetophenone
[0038] Dissolve 110 g of the above-ment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com