Compound, active energy ray curable composition, cured article thereof, printing ink, and inkjet recording ink
A compound and reactive compound technology, applied in printing, ink, application, etc., can solve problems such as poor photoinitiation ability and poor curing, and achieve the effect of fast curing speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0294] (Example 1) Synthesis of compound (5') [2-dimethylamino-1-(4-morpholinophenyl)-2-(4-carboxybenzyl) butane-1-one]
[0295] [Process 1]
[0296]
[0297] 121.8 g of aluminum chloride (anhydrous) and 300 mL of dehydrated dichloromethane were charged into a 1 L four-necked flask equipped with a stirrer, a thermometer, a nitrogen gas introduction tube, an alkali trap (Alkali Trapp) and a dropping funnel, under a nitrogen stream , using an ice bath for ice cooling. 200 g of 2-bromobutyryl bromide was added thereto. A mixed solution of 83.6 g of fluorobenzene and 100 mL of dehydrated dichloromethane was dropped into the previous flask over 20 minutes using a dropping funnel. After the dropwise addition was completed, the ice bath was removed, and stirring was continued for 2 hours as it was.
[0298] After the stirring was completed, the reaction solution was poured into 1 L of ice water, and the stirring was continued for 2 hours. After standing still, the liquid was s...
Embodiment 2
[0316] (Example 2) Compound (6') (2-Dimethylamino-1-(4-morpholinophenyl)-2-(4-(1-hydroxyl-1-oxopropane-2-yl) Synthesis of benzyl)butan-1-one)
[0317]
[0318] In step 1 of Example 1, 97.6 g of methyl 2-(4-bromomethyl)phenylpropionate (105) was used instead of 87.0 g of methyl 4-(bromomethyl)benzoate (103), except Otherwise, react according to the method described in Example 1 to synthesize compound (6') (2-dimethylamino-1-(4-morpholinophenyl)-2-(4-(1-hydroxyl-1 -Oxopropan-2-yl)benzyl)butan-1-one).
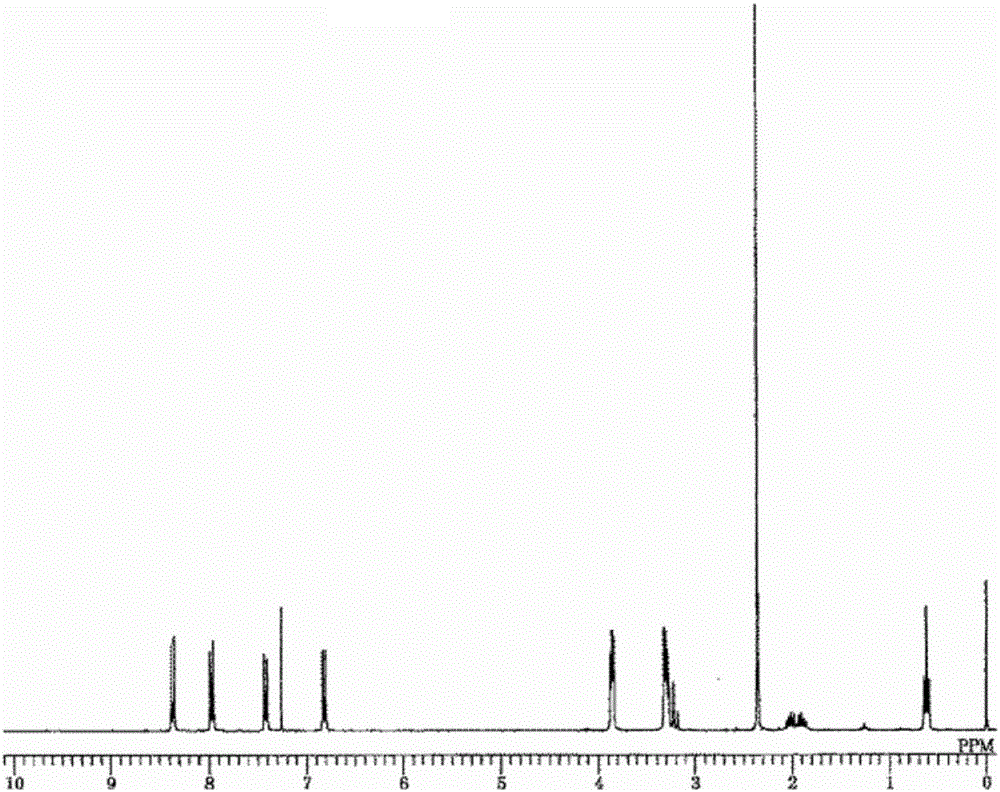
[0319] 1 H-NMR (CDCl 3 ): 0.66ppm (t, 3H, - CH 3 ), 1.46(d, 3H, CH- CH 3 ), 1.83ppm (m, 1H, - CH 2 -), 2.00ppm (m, 1H, - CH 2 -), 2.35(s, 6H, N- CH 3 ), 3.16(s, 2H, - CH 2 -Ph), 3.30ppm (m, 4H, morpholine), 3.67 (q, 1H, CH -CH 3 ), 3.84ppm (m, 4H, morpholine), 6.82ppm (d, 2H, aromatic), 7.39ppm (d, 2H, aromatic), 7.95ppm (d, 2H, aromatic), 8.35ppm (d , 2H, aromatic)
Embodiment 3
[0320] (Example 3) Compound (8') [2-methyldodecylamino-1-(4-morpholinophenyl)-2-(4-carboxybenzyl)butan-1-one] synthesis
[0321]
[0322] In step 1 of Example 1, 256.6 g of methyl dodecylamine was used instead of 789.9 g of 11% dimethylamine / ethanol solution, except that, according to the method described in Example 1, compound (8') was synthesized ( 2-methyldodecylamino-1-(4-morpholinophenyl)-2-(4-carboxybenzyl)butan-1-one).
[0323] 1 H-NMR (CDCl 3 ): 0.66ppm (t, 3H, - CH 3 ), 0.84ppm (t, 3H, - CH 3 ), 1.20ppm (m, 18H, - CH 2 -), 1.34ppm (m, 2H, N-CH 2 - CH 2 -CH 2 -), 1.83ppm (m, 1H, - CH 2 -), 2.00ppm (m, 1H, - CH 2 -), 2.30(s, 3H, N- CH 3 ), 2.40(m, 1H, N- CH 2 -), 2.60 (m, 1H, N- CH 2 -), 3.10(s, 2H, - CH 2-Ph), 3.30ppm (m, 4H, morpholine), 3.84ppm (m, 4H, morpholine), 6.82ppm (d, 2H, aromatic), 7.39ppm (d, 2H, aromatic), 7.95ppm (d, 2H, aromatic), 8.35ppm (d, 2H, aromatic)
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com