Enhancement of angiogenesis to grafts using cells engineered to produce growth factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
VEGF Secretion from Encapsulated CHO VEGF Cells
I. Material and Methods
Matrix:
A biological acellular collagen matrix was isolated from Swine kidneys. Swine kidneys were harvested, agitated in triton 100X (Sigma St. Louis, Mo.) and ammonium hydroxide (Sigma). The matrices were washed extensively with saline, and allowed to dry. The matrices were lyophilized and cut into 3×3 mm sections. Scanning electron microscopy confirmed the acellular nature of the collagen matrices.
Renal Cells:
Kidneys from 5 day old C57 black mice (Jackson labs; Bar Harbore, Me.) were harvested, minced, suspended in Keratinocyte SFM medium (Gibco BRL; New York, N.Y.) containing Collagenase / Dispase 1 mg / ml (Boehringer Mannheim; Mannheim, Germany) and agitated for one hour at 37° C. The mixed culture of renal cells was filtered serially through 104 μm sieves until all undigested tissue was removed. The filtrate was washed twice with medium and seeded on the collagen matrices at a density of 30×106 cells / m...
example 2
Genetically Engineering Muscle Cells to Transiently Produce VEGF
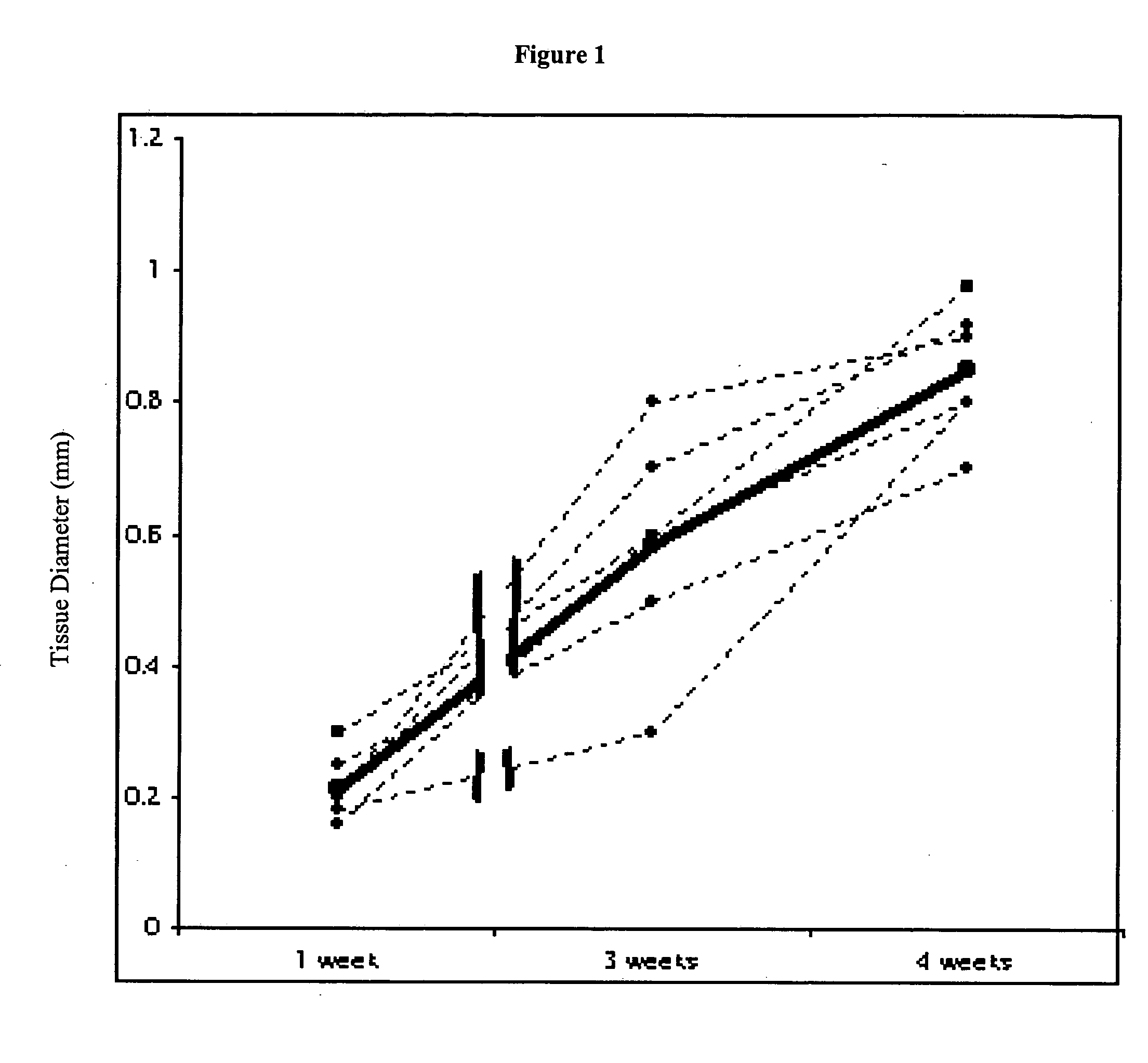
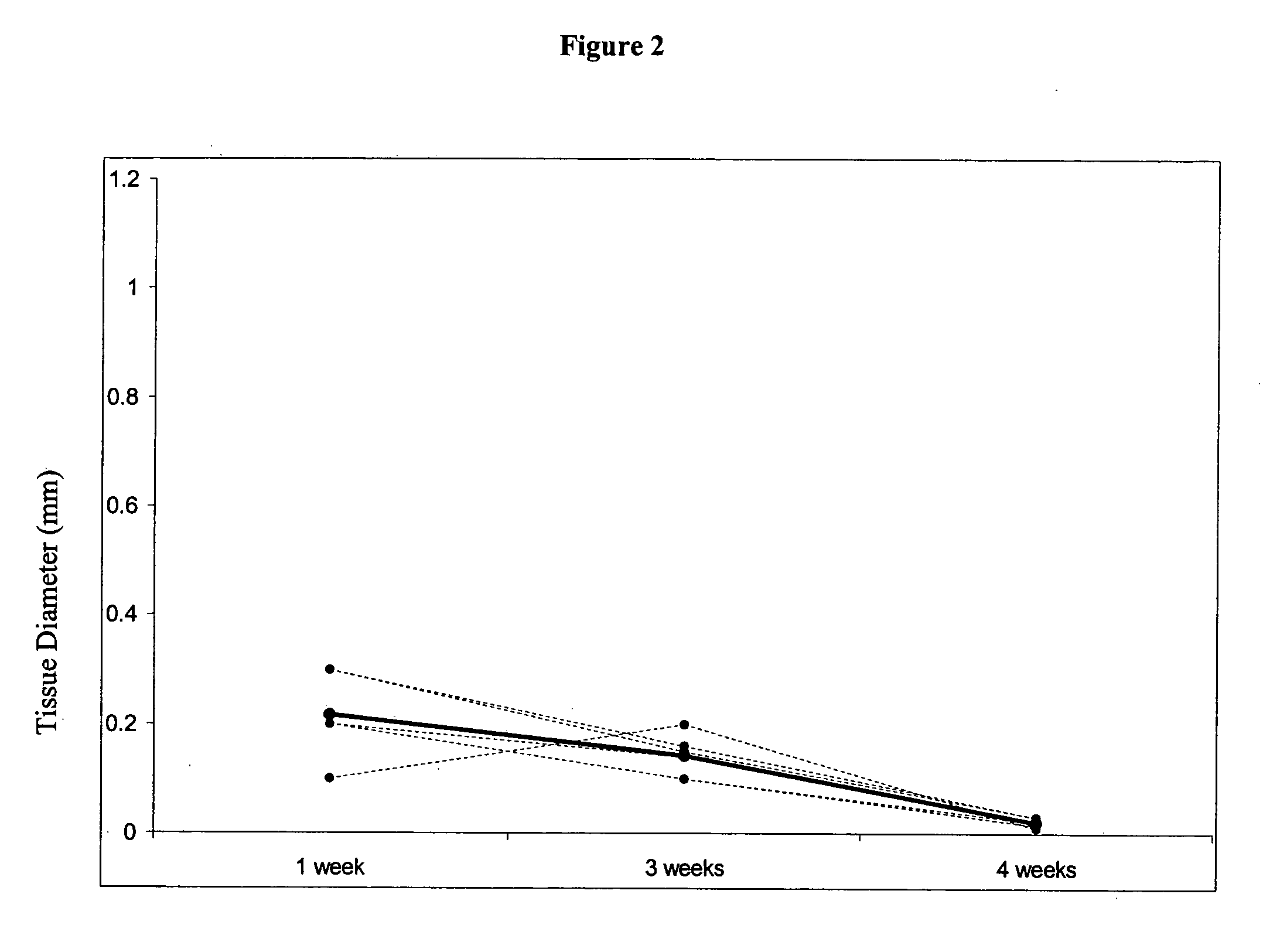
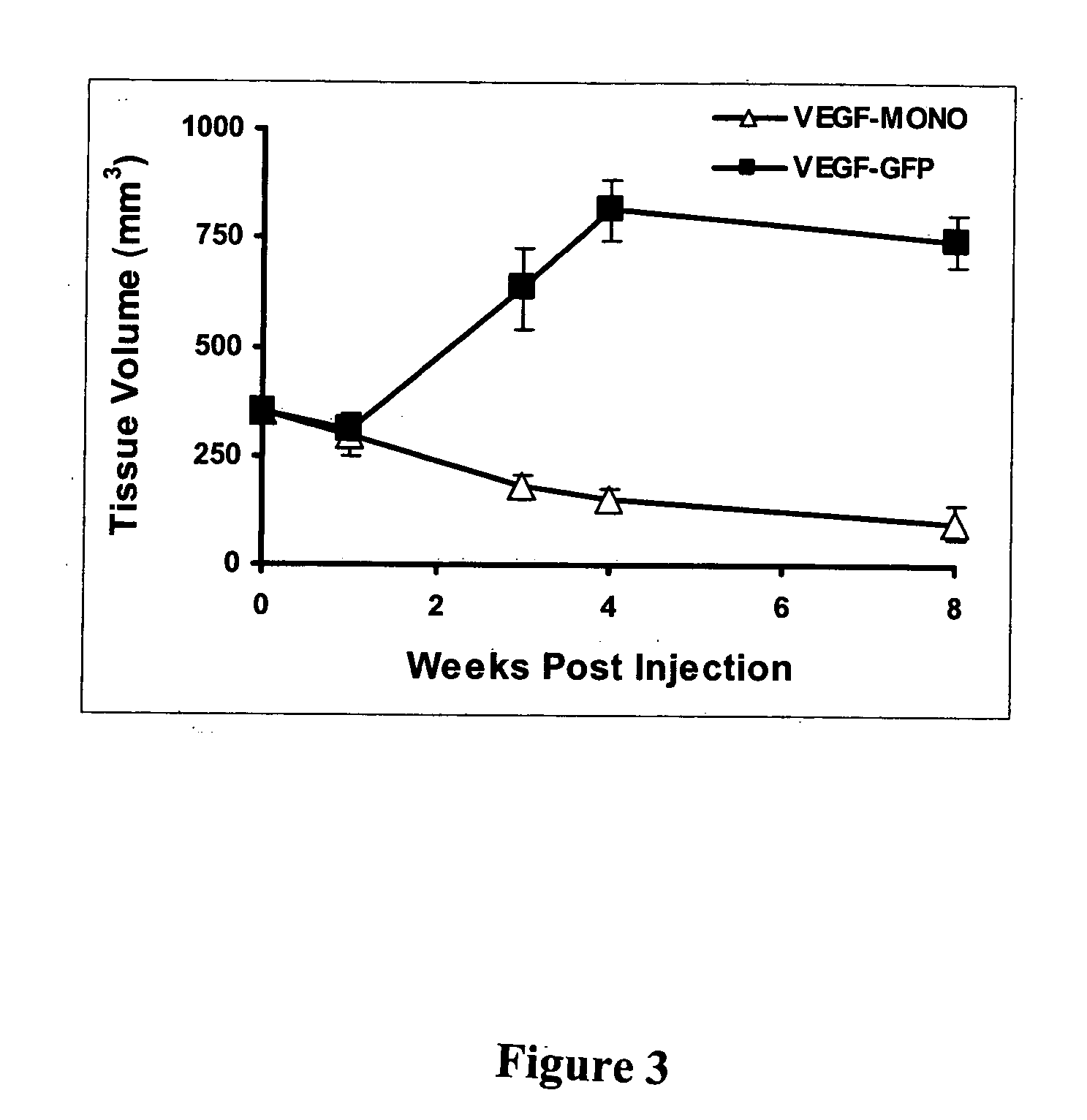
This study demonstrates that in vivo engineered muscle tissues improve their vascularity when VEGF gene modified muscle cells are used. Desmin positive myoblasts were obtained that formed spontaneously contractile myofibers by 2 weeks in vitro. VEGF and GFP expression in vitro was respectively assessed by Western blot analysis and fluorescence, showing that the myoblasts were successfully transfected. Analysis of the in vivo recovered engineered tissues with RT-PCR and fluorescence showed expression of VEGF and GFP proteins. Immunohistochemical analysis of the recovered tissues confirmed the muscle phenotype. Neovascularization and muscle tissue mass significantly increased with the functional VEGF-transfected cells compared with the non-functional VEGF-transfected cells.
Myoblasts Isolation:
All procedures were done in according with Children's Hospital Animal Care Committee protocols. Myoblasts were derived from s...
example 3
Muscle Formation, Neovascularization, and Volume Preservation in Engineered Tissues Using a Combination of VEGF and Endothelial Cells
This Example illustrates the methods of this invention by demonstrating tissue neovascularization following the implantation of myoblasts and vascular EC in bioengineered tissues. Dermal capillary-derived EC were used in this Example because they represent the same type of the EC in the mouse skin blood vessels that serve the source of EC for subcutaneous neovascularization. Incorporation of EC into the developing tissue had no major effect on tissue volumes of VEGF expressing and control myoblasts. This finding may be explained by the observation that the transplanted vascular EC were a small fraction of the vascular network of the newly formed tissues and the majority was the host (i.e., mouse) EC. However, incorporation of vascular EC enhanced and prolonged MyoD expression and thus, contributed to muscle differentiation of the engineered tissue. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Vascularization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com