Preparation technology of high purity 3-hydroxyacetophenone
A technology for hydroxyacetophenone and preparation process, which is applied in the preparation of carbon-based compounds, preparation of organic compounds, organic chemistry and other directions, can solve the problems of low product purity, low product yield and high production cost, and achieves high product purity, The effect of high product yield and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
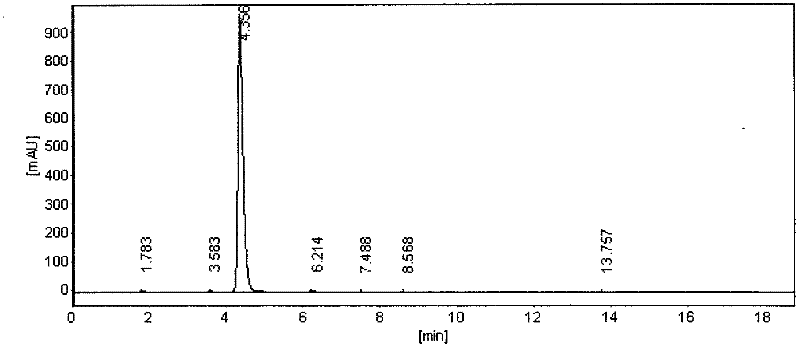
Image
Examples
Embodiment 1
[0014] Example 1: Add 36 parts by weight of 3-nitroacetophenone, 0.03 parts by weight of methanol and 36 parts by weight of water into the reaction kettle, and then slowly add 22 parts by weight of iron powder to react for 10 hours after heating to 95°C After the filtrate enters the receiving tank, it is gradually cooled to 30°C after 10 hours. The precipitate is centrifuged to remove water and 3-aminoacetophenone is obtained (the centrifugal water is fully recovered and recycled as the reaction water for the next feeding ); Pour the obtained 3-aminoacetophenone into the diazonium kettle, add 8 parts by weight of 98% sulfuric acid, cool to 10°C, and then slowly add 8 parts by weight of sodium nitrite aqueous solution to the diazonium kettle After adding in 15 hours, 3-diazosulfate acetophenone is obtained; add 19 parts by weight of water to the hydrolysis kettle, and then increase the temperature to 95° C. to obtain 3-diazosulfate acetophenone Slowly added to the hydrolysis ket...
Embodiment 2
[0015] Example 2: Add 38 parts by weight of 3-nitroacetophenone, 0.03 parts by weight of methanol and 37 parts by weight of water into the reaction kettle, and slowly add 21 parts by weight of iron powder to react for 10 hours after heating to 95°C After the filtrate enters the receiving tank, it is gradually cooled to 30°C after 10 hours. The precipitate is centrifuged to remove water and 3-aminoacetophenone is obtained (the centrifugal water is fully recovered and recycled as the reaction water for the next feeding ); Pour the obtained 3-aminoacetophenone into the diazonium kettle, add 6 parts by weight of 98% sulfuric acid, cool to 5°C, and then slowly add 7 parts by weight of sodium nitrite aqueous solution to the diazonium kettle After adding in 15 hours, 3-diazosulfate acetophenone was prepared; 18 parts by weight of water was added to the hydrolysis kettle, and then the temperature was raised to 95°C, and the obtained 3-diazosulfate acetophenone The ketone was slowly add...
Embodiment 3
[0016] Example 3: Add 37 parts by weight of 3-nitroacetophenone, 0.04 parts by weight of methanol and 38 parts by weight of water into the reaction kettle, and slowly add 20 parts by weight of iron powder to react for 10 hours after heating to 95°C After the filtrate enters the receiving tank, it is gradually cooled to 30°C after 10 hours. The precipitate is centrifuged to remove water and 3-aminoacetophenone is obtained (the centrifugal water is fully recovered and recycled as the reaction water for the next feeding ); Pour the obtained 3-aminoacetophenone into the diazonium kettle, add 7 parts by weight of 98% sulfuric acid, cool to 8°C, and then slowly add 6 parts by weight of sodium nitrite aqueous solution to the diazonium kettle After adding in 15 hours, 3-diazosulfate acetophenone is obtained; add 17 parts by weight of water to the hydrolysis kettle, and then increase the temperature to 95° C. to obtain 3-diazosulfate acetophenone Slowly add to the hydrolysis kettle, aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com