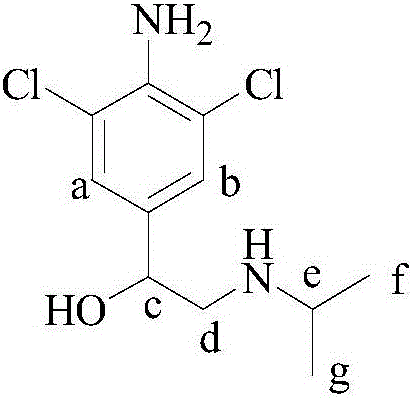
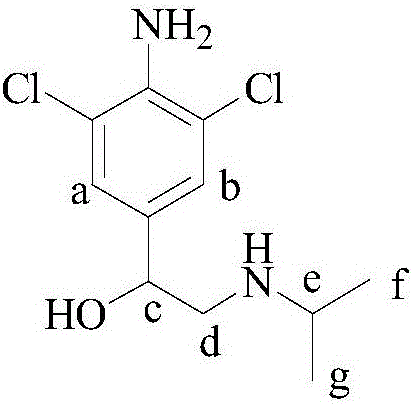
Stable isotope labeling Clenproperol compound and synthesis method thereof
A technology of stable isotope and synthesis method, which is applied in the field of stable isotope-labeled Clempro compound and its synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] A stable isotope labeled cromppro-D 2 The preparation method comprises the following steps:
[0158] 1. Stable isotope labeling 3,5-dichloro-4-aminoacetophenone-D 2 preparation of
[0159] Add 16.5g 2,6-dichloroaniline-D in a 250mL three-necked flask 3 , add 50mL of dichloromethane and stir to disperse, then add 27g of aluminum trichloride, add 11.8g of acetyl chloride dropwise under temperature control at 0°C, after the dropwise addition, raise the temperature and reflux to react for 4 hours, after acidification, filter with suction and wash with water to obtain 17.3g of light yellow 3,5-dichloro-4-aminoacetophenone-D 2 , yield 85.1%, HPLC detection, purity 99.5%; mass spectrometry detection, abundance 99.5atom%D.
[0160] 2. Stable isotope labeling of 3,5-dichloro-4-amino-α-bromoacetophenone-D 2 preparation of
[0161] Add 10.1g of 3,5-dichloro-4-aminoacetophenone-D into a 100mL three-neck flask 2 , add 20mL deuterated chloroform, copper bromide 22.5g, heat up ...
Embodiment 2
[0167] A stable isotope labeled cromppro-D 1 The preparation method comprises the following steps:
[0168] 1, Preparation of 3,5-dichloro-4-aminoacetophenone
[0169] Add 8.3g of 2,6-dichloroaniline to a 250mL three-neck flask, add 50mL of chloroform and stir to disperse, add 30g of ferric chloride, add 8.8g of acetyl chloride dropwise at a temperature of 0°C, and react at room temperature for 6 hours, after acidification, suction filtration and water washing to obtain 9.0 g of light yellow 3,5-dichloro-4-aminoacetophenone with a yield of 88.6%, and a purity of 99.5% by HPLC detection.
[0170] 2. Preparation of 3,5-dichloro-4-amino-α-bromoacetophenone
[0171] Add 5.1g of 3,5-dichloro-4-aminoacetophenone into a 100mL three-neck flask, add 20mL of chloroform, add 10g of liquid bromine dropwise, react at room temperature for 3 hours after the addition, stop the reaction, neutralize, and recover the solvent after washing with water 6.7 g of a light yellow solid was obtained,...
Embodiment 3
[0177] A stable isotope labeled cromppro-D 7 The preparation method comprises the following steps:
[0178] 1, Preparation of 3,5-dichloro-4-aminoacetophenone
[0179] Add 16.2g of 2,6-dichloroaniline into a 250mL three-neck flask, add 50mL of chloroform and stir to disperse, then add 20g of boron trifluoride, add 15.3g of acetyl chloride dropwise at 0°C, and react at room temperature for 3 After acidification, suction filtration and water washing gave 17.7g of light yellow 3,5-dichloro-4-aminoacetophenone with a yield of 86.9%. The purity was 99.5% by HPLC detection.
[0180] 2. Preparation of 3,5-dichloro-4-amino-α-bromoacetophenone
[0181] Add 10.2g of 3,5-dichloro-4-aminoacetophenone into a 100mL three-neck flask, add 20mL of dichloromethane, add 15g of NBS, react at room temperature for 5 hours after the addition, stop the reaction, and recover the solvent after washing with water to obtain 13.6g Pale yellow solid, yield 96.3%, HPLC detection, purity 99.0%.
[0182] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com