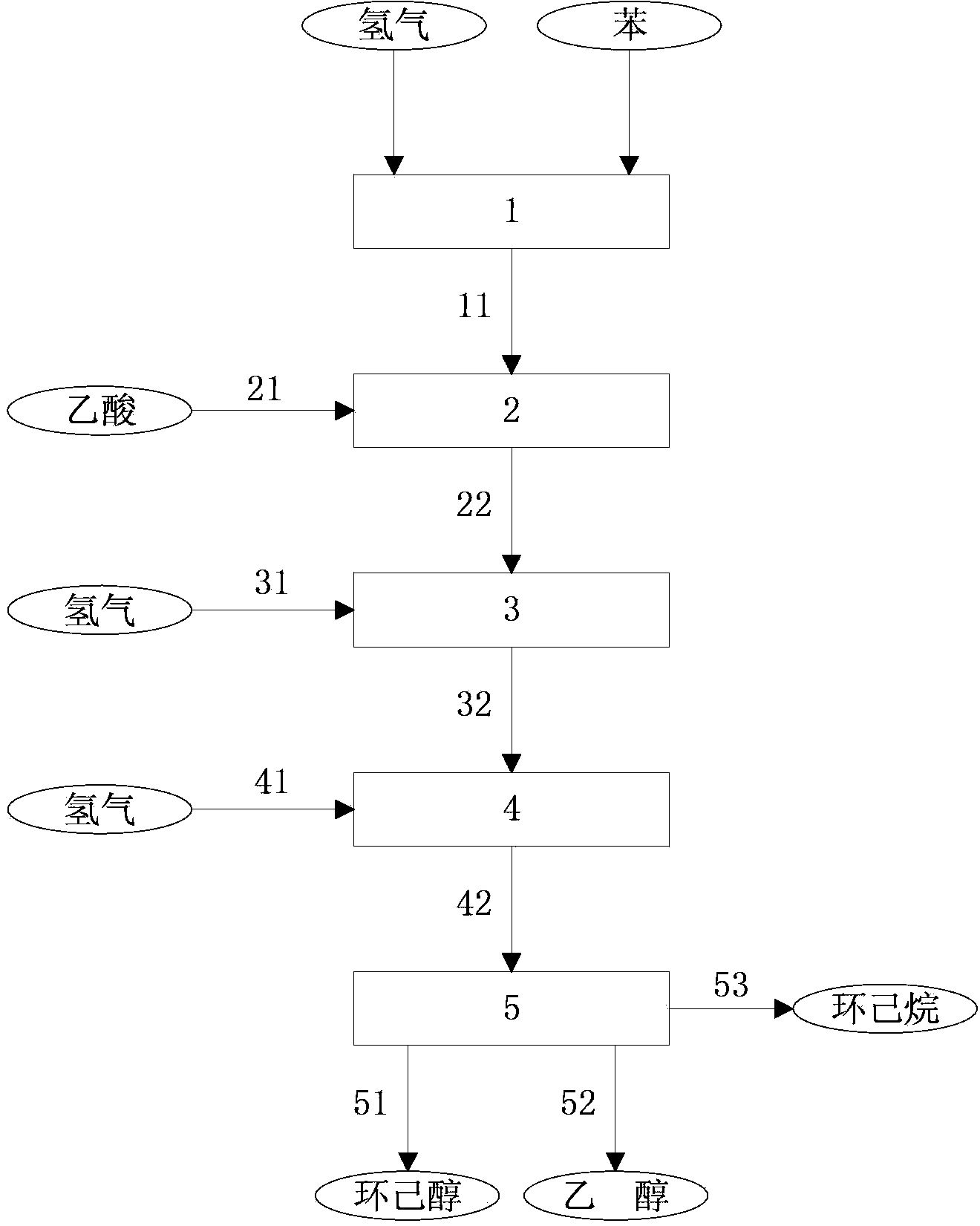
Method for producing cyclohexanol
A technology of cyclohexanol and cyclohexene, applied in the field of cyclohexanol production, can solve the problem of no cyclohexanol and ethanol and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] This example illustrates the method for the selective hydrogenation of benzene to cyclohexene.
[0071] Benzene and hydrogen are injected into the hydrogenation reactor filled with ruthenium particle catalyst at a molar ratio of 1:3, and the benzene hydrogenation reaction is carried out under the conditions of a reaction temperature of 135°C, a pressure of 4.5MPa, and a residence time of 15min. After the reaction product is separated from the hydrogen , Collect the liquid product and run continuously for 1000h. After the test, the collected liquid product was analyzed by gas chromatography, and its composition was: 53.3% benzene, 35.4% cyclohexene, and 11.3% cyclohexane.
Embodiment 2
[0073] 100mL of macroporous strongly acidic hydrogen-type ion exchange resin (synthesized in the laboratory according to the classic literature method, suspension copolymerization of styrene solution containing 15% divinylbenzene to make white balls, and then sulfonated with concentrated sulfuric acid, measured Its exchange capacity is 5.2mmolH + / g dry basis) into the middle of a stainless steel tube reactor with a jacket of φ32×4×1000mm, and a certain amount of quartz sand is filled at both ends. The raw materials of acetic acid and cyclohexene (53.3% benzene, 35.4% cyclohexene, and 11.3% cyclohexane) are pumped into the reactor by a metering pump at a certain flow rate for reaction, and heat is introduced into the external jacket of the reaction tube. Water is used to control the reaction temperature, and the reactor pressure is controlled through the reactor outlet back pressure valve. The outlet product of the reactor was sampled by an online sampling valve for online ch...
Embodiment 3
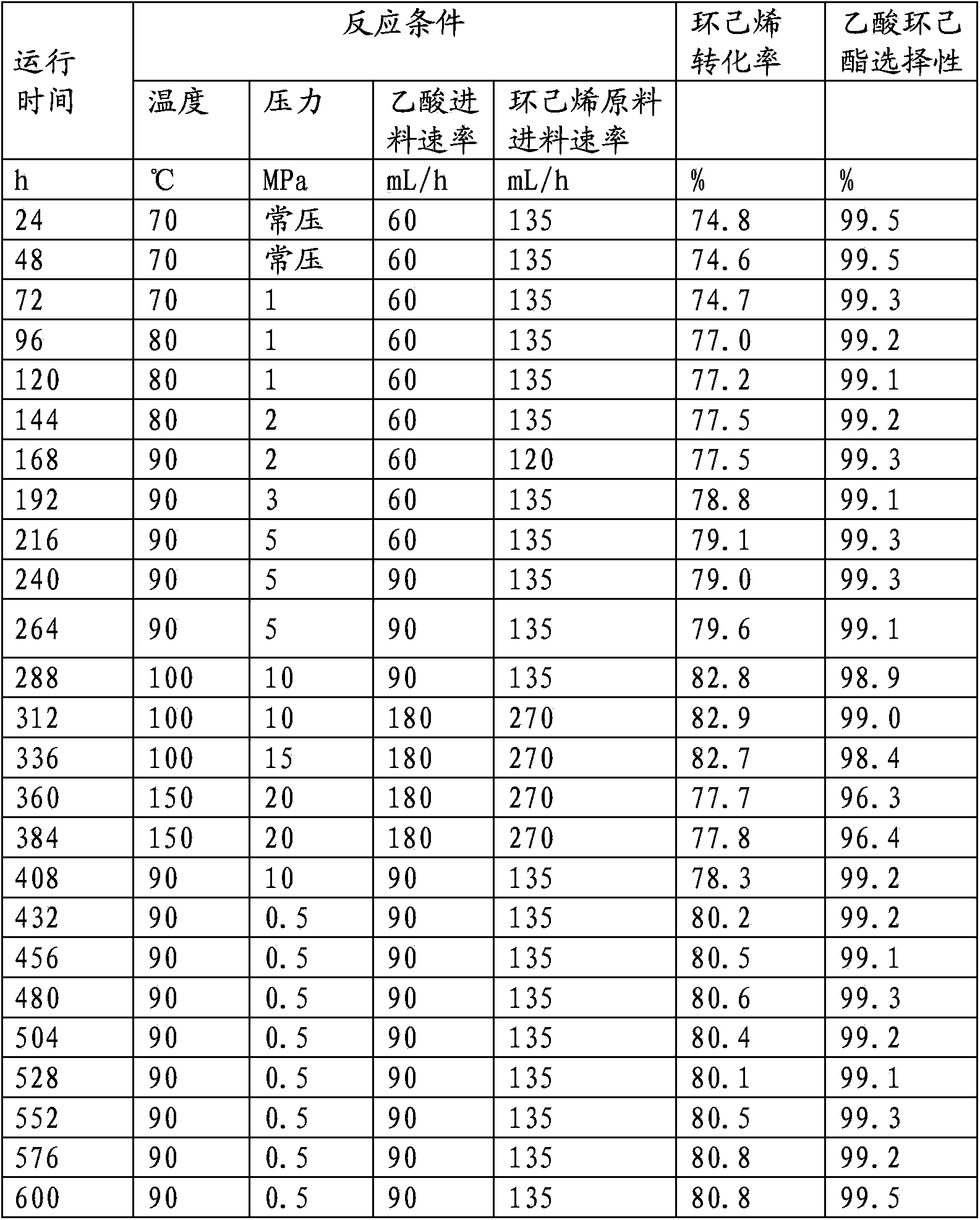
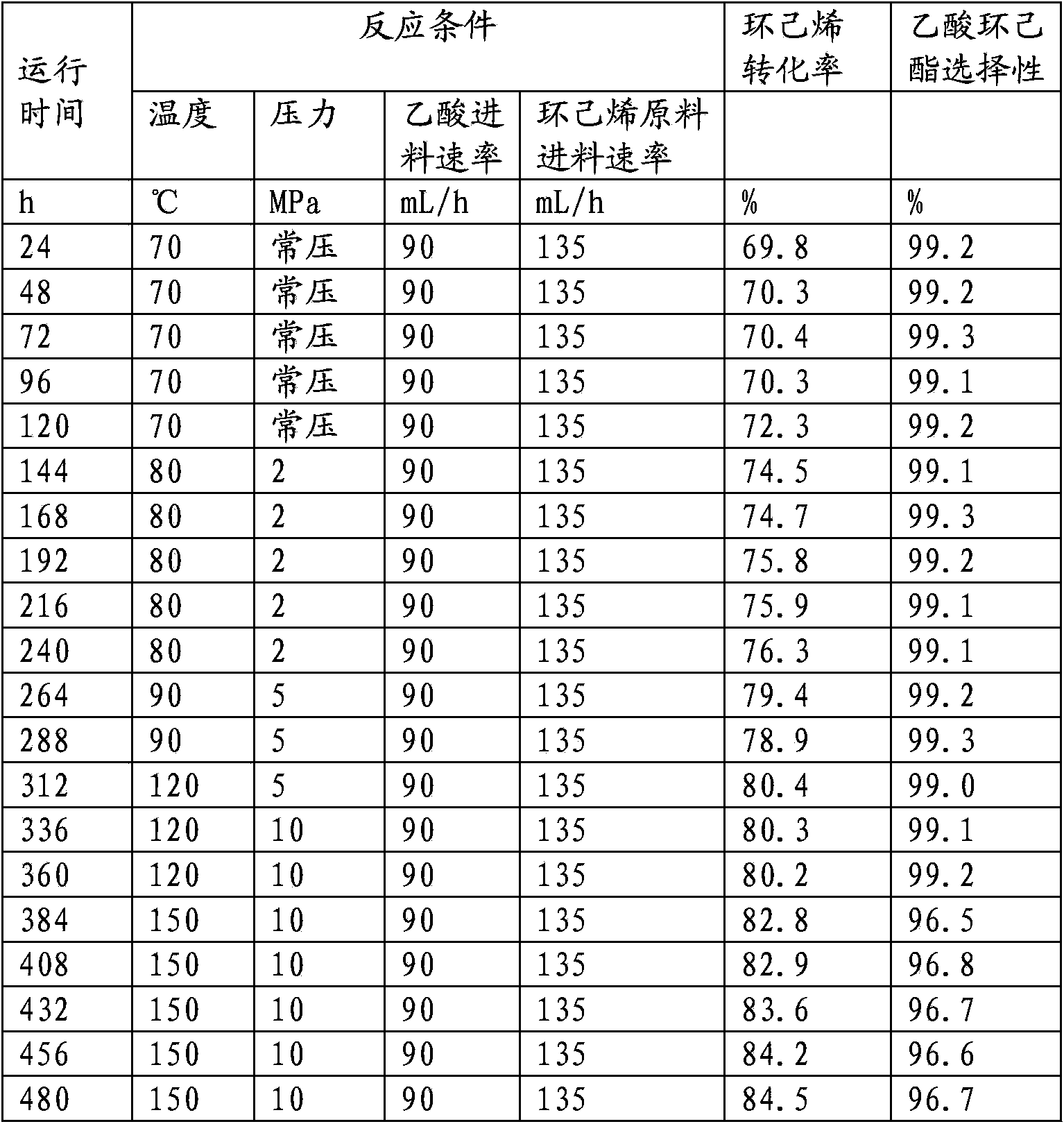
[0078] The test device, method and raw materials are the same as in Example 2, except that the catalyst is a phosphorus-modified Hβ molecular sieve catalyst (the Hβ molecular sieve with a silicon-aluminum ratio of 50 is modified with 85% phosphoric acid, and then kneaded with alumina to form , obtained by drying at 120°C and roasting at 500°C, with a phosphorus content of 2%). The reaction conditions and results are shown in Table 2. It can be seen from Table 2 that the reaction conversion rate of cyclohexene and acetic acid is greater than 80%, the selectivity of ester product is greater than 99%, and the catalyst activity and selectivity are stable after running for 480 hours.
[0079] Table 2 Hβ molecular sieve catalyst catalyzed acetic acid and cyclohexane / cyclohexene / phenyl esterification test data
[0080]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com