Monatomic iron-sulfur-nitrogen co-doped carbon aerogel electrocatalyst and preparation method and application thereof
A carbon airgel and electrocatalyst technology, applied in the field of electrocatalysis, can solve the problems of unclear catalytic active sites and low ammonia production rate, and achieve the effects of improving catalytic reaction rate, low preparation cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation process of monoatomic iron-sulfur-nitrogen co-doped carbon airgel electrocatalyst:
[0038] (1) Dissolve 4g of urea in 200mL of deionized water, heat the solution to 80°C and stir for 1 hour; add 4g of carrageenan and 0.5g of ferric nitrate nonahydrate, keep stirring at 80°C until the carrageenan and ferric nitrate are completely dissolved , to obtain a mixed solution;
[0039](2) The mixed solution obtained in step (1) is naturally cooled to room temperature, and freeze-dried to form an iron-urea-carrageenan mixture aerogel; the freeze-drying time is 24h;
[0040] (3) Calcinate the airgel obtained in step (2) at 900°C for 2h under a nitrogen atmosphere, put the obtained sample in 2M hydrochloric acid solution and etch for 24h, then wash it with deionized water until neutral, and vacuum at 80°C After drying, the carbon airgel electrocatalyst was obtained, which was designated as Fe-S-N-900.
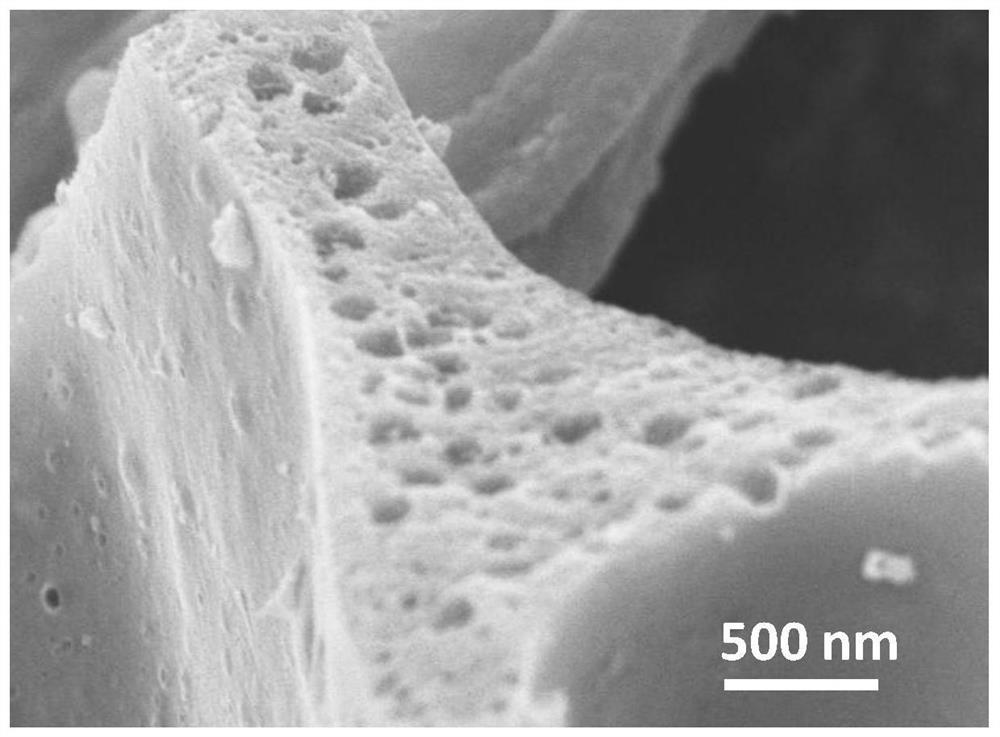
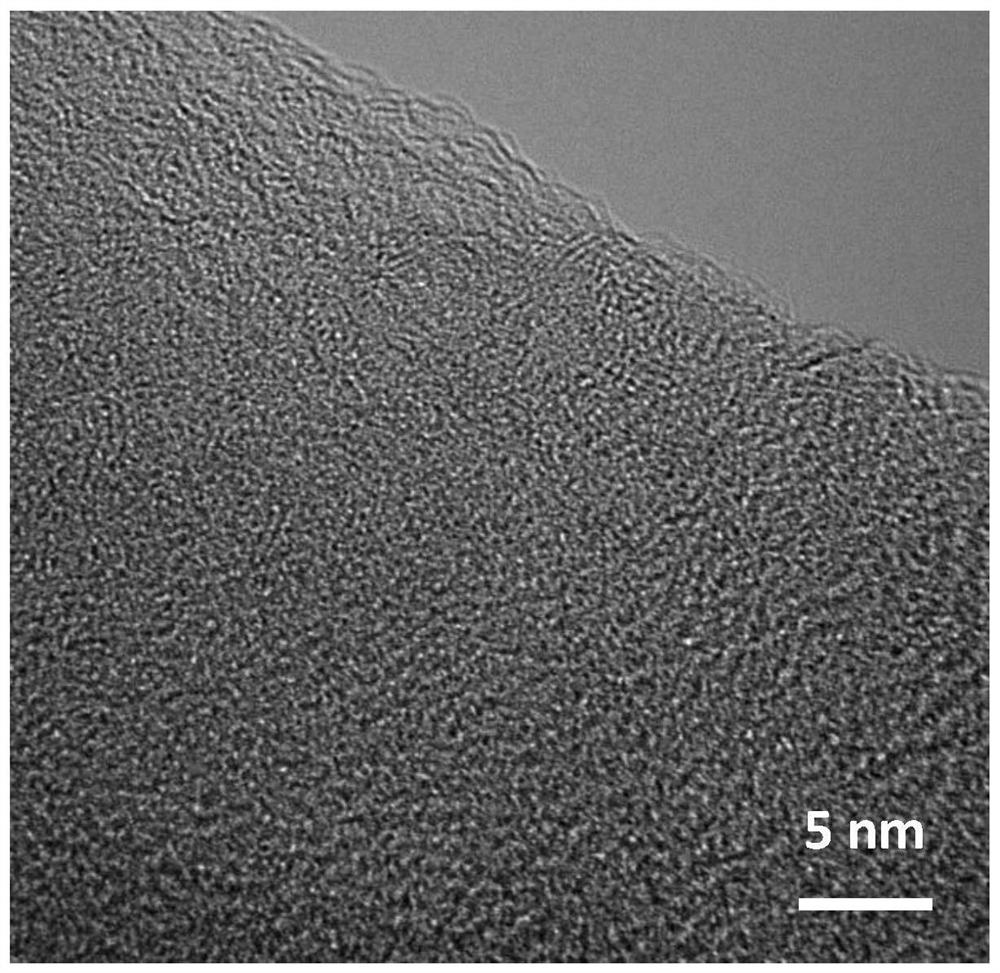
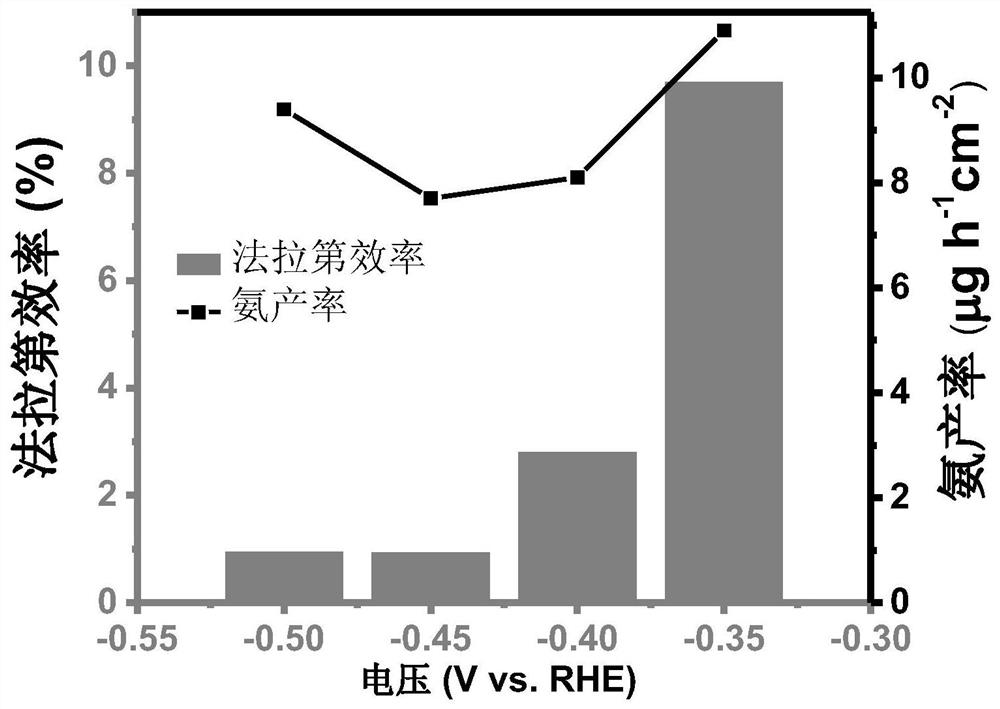
[0041] like figure 1 As shown, the prepared Fe-S-N-900 catalyst...
Embodiment 2~4
[0043] According to the preparation process of Example 1, the calcination temperature in step (3) was changed to 800 ° C, 1000 ° C and 1100 ° C, respectively, to obtain carbon airgel electrocatalysts, respectively expressed as Fe-S-N-800, Fe-S-N-1000 and Fe-S-N-1100.
[0044] Iron atoms (0.54wt%, 0.84wt%, 0.74wt%, 0.48wt%) of the prepared catalyst (Fe-S-N-800, Fe-S-N-900, Fe-S-N-1000, Fe-S-N-1000), S (2.5wt%, 4.04wt%, 4.9wt%, 5.59wt%) and N (9.36wt%, 3.93wt%, 2.38wt%, 2.23wt%), the specific surface area is (197m 2 g -1 ,547m 2 g -1 , 379m 2 g -1 , 249m 2 g -1 ), pore volume (0.04cm 3 g -1 ,0.1cm 3 g -1 , 0.08cm 3 g -1 , 0.05cm 3 g -1 ) pore size (8.7nm, 7.7nm, 7.5nm, 4.3nm).
Embodiment 5
[0046] Preparation process of monoatomic iron-sulfur-nitrogen co-doped carbon airgel electrocatalyst:
[0047] (1) Dissolve 4g of urea in 200mL of deionized water, heat the solution to 80°C and stir for 1 hour; add 4g of carrageenan, keep stirring at 80°C until the carrageenan and ferric nitrate are completely dissolved to obtain a mixed solution;
[0048] (2) The mixed solution obtained in step (1) is naturally cooled to room temperature, and freeze-dried to form an iron-urea-carrageenan mixture aerogel; the freeze-drying time is 24h;
[0049] (3) Calcinate the airgel obtained in step (2) at 900°C for 2h under a nitrogen atmosphere, put the obtained sample in 2M hydrochloric acid solution and etch for 24h, then wash it with deionized water until neutral, and vacuum at 80°C After drying, the carbon airgel electrocatalyst was obtained, which was designated as S-N-900.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum power density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com