CuAu bimetallic catalyst, and preparation method and application of CuAu bimetallic catalyst
A bimetallic catalyst, metal ammonia technology, applied in molecular sieve catalysts, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve the problems of uneven distribution of active component copper, poor catalytic activity, poor catalyst stability, etc. utilization rate and the effect of improving catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
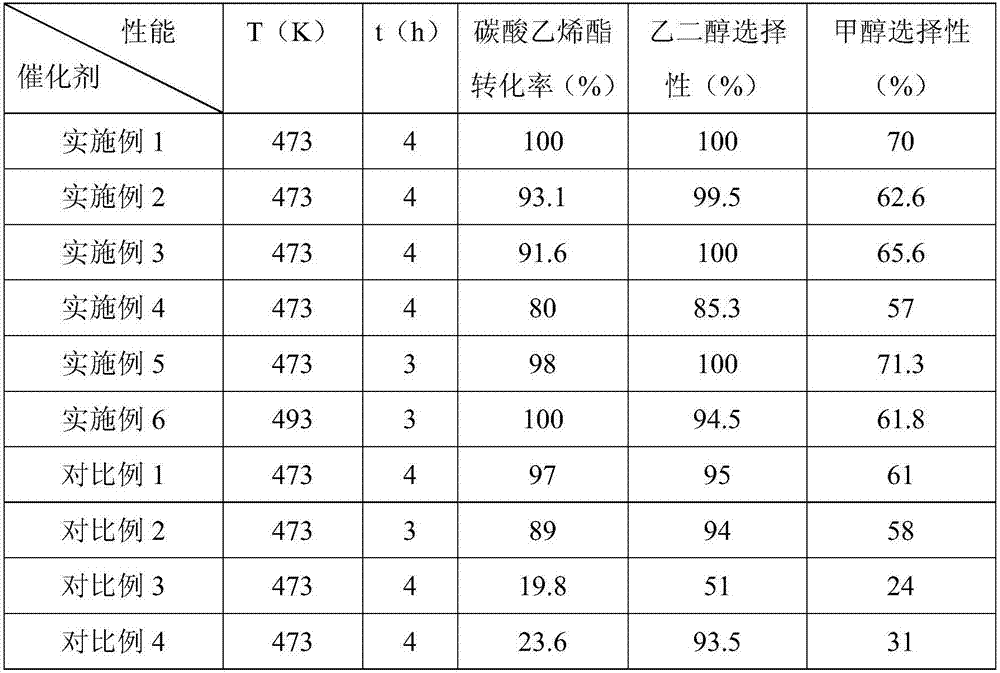
Embodiment 1
[0059] Preparation of CuAu bimetallic catalyst by ammonia distillation method:
[0060] (1) At room temperature, 1.1521g of Cu(NO 3 ) 2 ·3H 2 O with 0.0628 g of HAuCl 4 4H 2 O was dissolved in 50 mL of deionized water, and ammonia water was added to adjust the pH to 10 under stirring, and the stirring was continued for 10 min to obtain a metal ammonia complex solution;
[0061] (2) Add 2.67g of SBA-15 carrier to the metal ammonia complex solution obtained in step (1), add ammonia water to adjust the pH to 11.5, and stir for 4 hours to obtain a mixed solution;
[0062] (3) Heat the mixed solution obtained in step (2) to 80°C to remove ammonia by evaporation, stop heating after the pH drops to 6-7, filter the obtained mixed solution, and deionize the obtained precipitate with deionized water and ethanol After washing and filtering, drying at 80°C for 12 hours, a solid complex was obtained;
[0063] (4) Calcining the solid composite obtained in step (3) at 400° C. in air fo...
Embodiment 2
[0066] Preparation of CuAu bimetallic catalyst by ammonia distillation method:
[0067] (1) At room temperature, 1.1521g of Cu(NO 3 ) 2 ·3H 2 O with 0.0941 g of HAuCl 4 4H 2 O was dissolved in 50 mL of deionized water, and ammonia water was added to adjust the pH to 9.5 under stirring, and the stirring was continued for 10 min to obtain a metal ammonia complex solution;
[0068] (2) Add 2.655 g of SBA-15 carrier to the metal ammonia complex solution obtained in step (1), add ammonia water to adjust the pH to 12, and stir for 6 hours to obtain a mixed solution;
[0069] (3) Heat the mixed solution obtained in step (2) to 100°C to remove ammonia by evaporation, stop heating after the pH drops to 6-7, filter the obtained mixed solution, and deionize the obtained precipitate with deionized water and ethanol After washing and filtering, drying at 100°C for 8 hours, a solid complex was obtained;
[0070] (4) Calcining the solid composite obtained in step (3) at 380° C. for 4.5...
Embodiment 3
[0073] Preparation of CuAu bimetallic catalyst by ammonia distillation method:
[0074] (1) At room temperature, 1.1521g of Cu(NO 3 ) 2 ·3H 2 O with 0.1255 g of HAuCl 4 4H 2 O was dissolved in 50 mL of deionized water, and ammonia water was added to adjust the pH to 9 under stirring, and the stirring was continued for 10 min to obtain a metal ammonia complex solution;
[0075] (2) Add 2.64g of SBA-15 carrier to the metal ammonia complex solution obtained in step (1), add ammonia water to adjust the pH to 12.5, and stir for 2.5h to obtain a mixed solution;
[0076] (3) Heat the mixed solution obtained in step (2) to 120°C to remove ammonia by evaporation, stop heating after the pH drops to 6-7, filter the obtained mixed solution, and deionize the obtained precipitate with deionized water and ethanol After washing and filtering, drying at 90°C for 24 hours, a solid complex was obtained;
[0077] (4) Calcining the solid composite obtained in step (3) at 450° C. for 3 h in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com