Method for synthesizing indiplon
A synthetic method, the technology of indiplon, is applied in the new synthetic field of indiplon, which can solve the problems of harsh reaction conditions, long reaction time, expensive reagents, etc., and achieve short reaction time, low synthesis cost and excellent yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
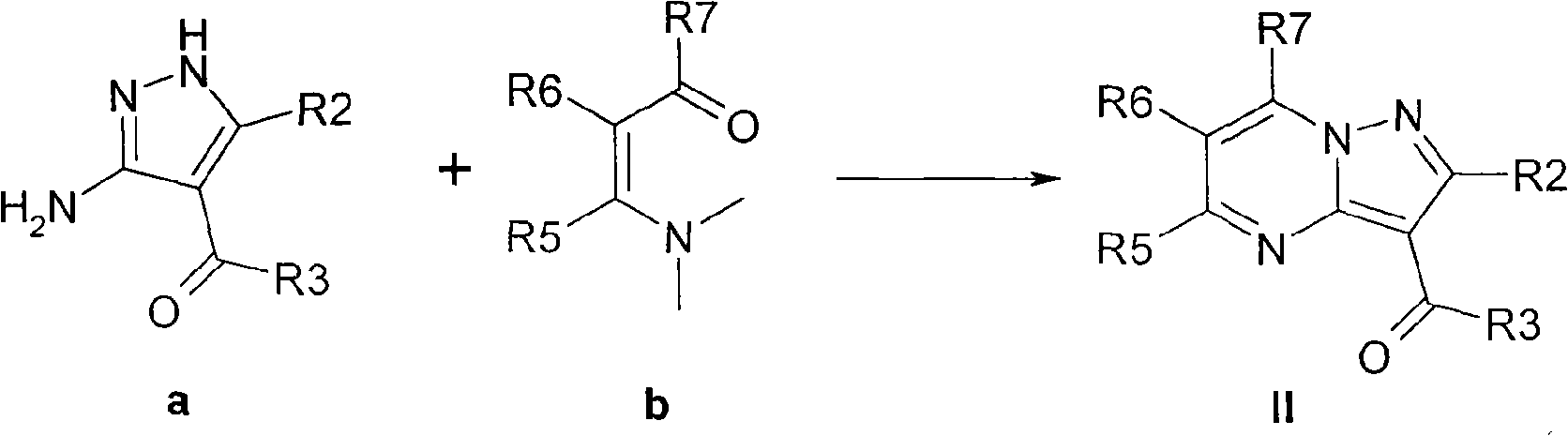
preparation example Construction
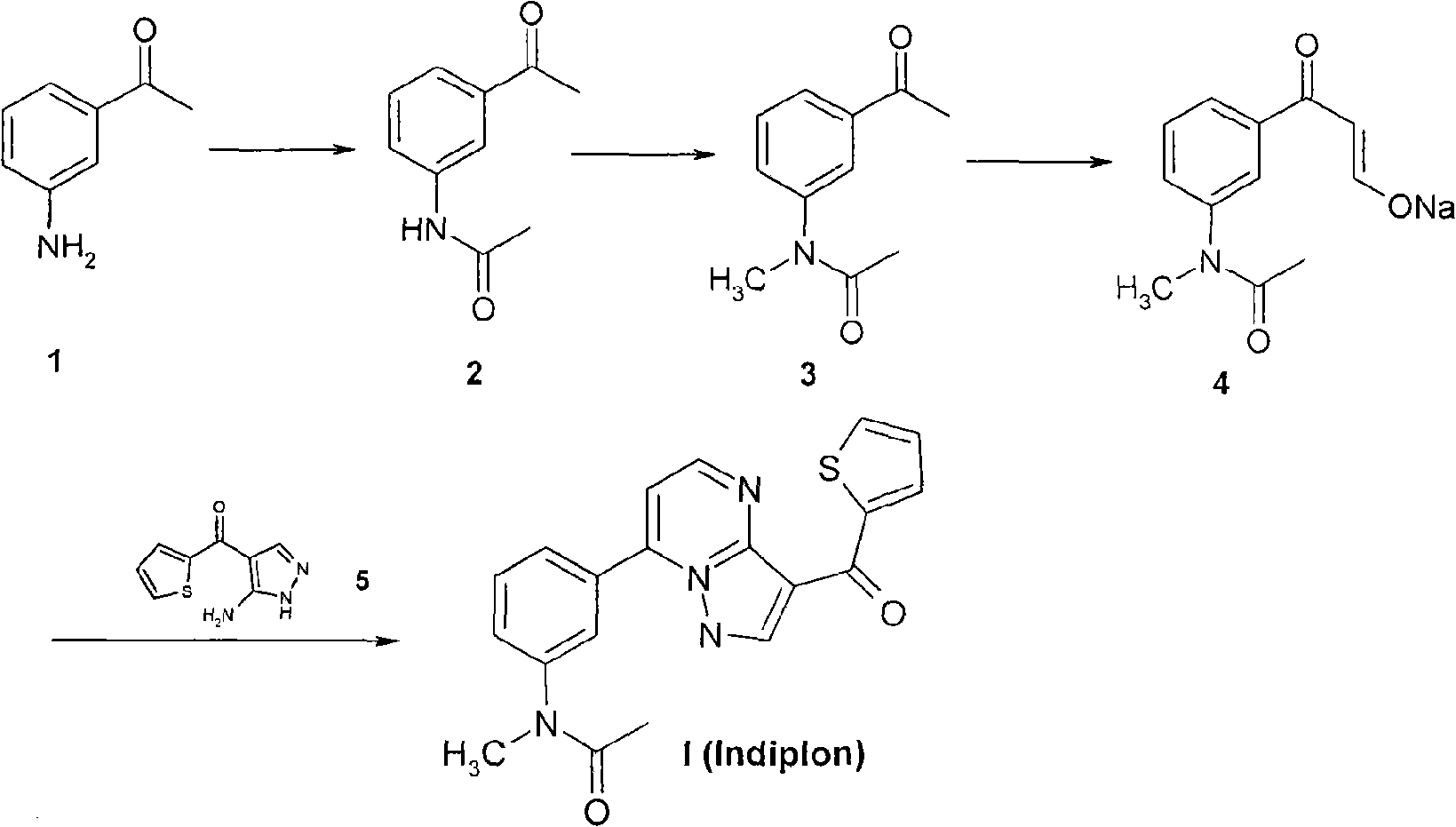
[0067] The method of the present invention is the preparation of indiplon:
[0068] (1) 3-aminoacetophenone at 0~room temperature, slowly add acetyl anhydride dropwise, react for 2~5 hours to convert into 3-acetamidoacetophenone, remove unreacted acetic acid with a large amount of water, use ethanol or acetic acid The pure product was obtained by recrystallization of ethyl ester; the yield was 90-99%.
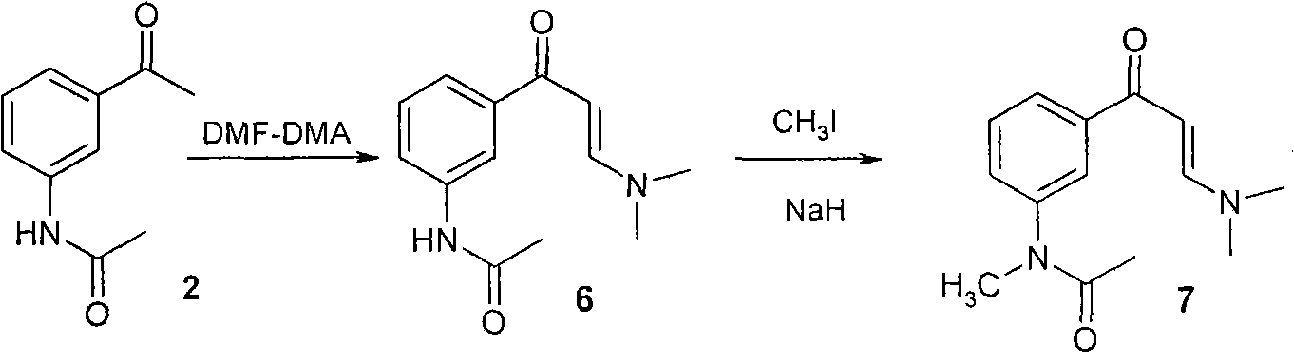
[0069] (2) 3-Acetamidoacetophenone is dissolved in tetrahydrofuran or toluene, first reacted with powdered potassium hydroxide, and then reacted with methyl iodide for 5 to 12 hours to transform N-methyl-N-(3-acetylbenzene base) acetamide, filter off the inorganic salt, wash with water, and remove traces with toluene to obtain the product with a yield of 85-95%. The product can be directly used in the next reaction without further purification.
[0070] (3) N-methyl-N-(3-acetylphenyl)acetamide is dissolved in alcohol, first reacted with sodium ethoxide, and then reacted with ...
Embodiment 1
[0087] Example 1 Preparation of 3-acetamidoacetophenone
[0088]
[0089] Add 318g of m-aminoacetophenone (2.356mol) in a 1000ml Erlenmeyer flask, slowly add 166ml of acetic anhydride (2.452mol) dropwise within 2 hours under ice-cooling and stirring, and a large amount of solids precipitate out. TLC showed that the starting material disappeared, and the reaction mixture was poured into 2500 ml of ice water, filtered with suction, washed with distilled water until neutral, and dried to obtain 381.6 g of light yellow solid. Melting point: 125.6.~127.3°C; heat to dissolve with 2000ml ethyl acetate, filter out impurities while hot, and the filtrate naturally cools down to room temperature. A large amount of solid was precipitated, filtered with suction, washed with ice ethyl acetate, and dried to obtain 3-acetamidoacetophenone 351.9 as colorless granular crystals, with a yield of 96%. Melting point: 127.0.~128.4°C.
[0090] The product is detected by nuclear magnetic resonan...
Embodiment 2
[0091] Example 2 Preparation of N-methyl-N-(3-acetylphenyl)acetamide
[0092]
[0093] In a 250ml round-bottomed flask, add a magnet, dry it with a vacuum flame, cool to room temperature, and pass through N 2 , 11.128 grams of powdered KOH (0.199 mol) were added rapidly, 160 ml of a tetrahydrofuran solution containing 17.295 grams of 3-acetamidoacetophenone (0.098 mol) and 15 ml of methyl iodide (0.244 mol) were added, and stirred overnight at room temperature. Inorganic salts were filtered off, washed with 3×30ml of water, and concentrated. A small amount of water was removed from 40 ml of toluene to obtain 17.593 g of N-methyl-N-(3-acetylphenyl)acetamide as a colorless oil, with a yield of 94%.
[0094] The product is detected by nuclear magnetic resonance, and the data are as follows: 1 HNMR (400MHz, CDCl 3 )δ ppm : 1.872(s, 3H, COC H 3 ), 2.625 (s, 3H, NCOCH 3 ), 3.280 (s, 3H, NCH 3 ), 7.402(d, 2H, J=7.6Hz, 2'-H), 7534(t, 1H, J=7.6Hz, 3'-H), 7.7.791(s, H, 6'-H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com