Quinoline derivative efficient catalytic synthesis method
A technology for quinolines and derivatives, which is applied in the field of efficient catalytic synthesis of quinoline derivatives, can solve the problems of low utilization rate of reaction raw materials, difficult biodegradation of catalysts, complicated operation processes, etc., and achieves easy industrialized large-scale production, The effect of many cycles and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
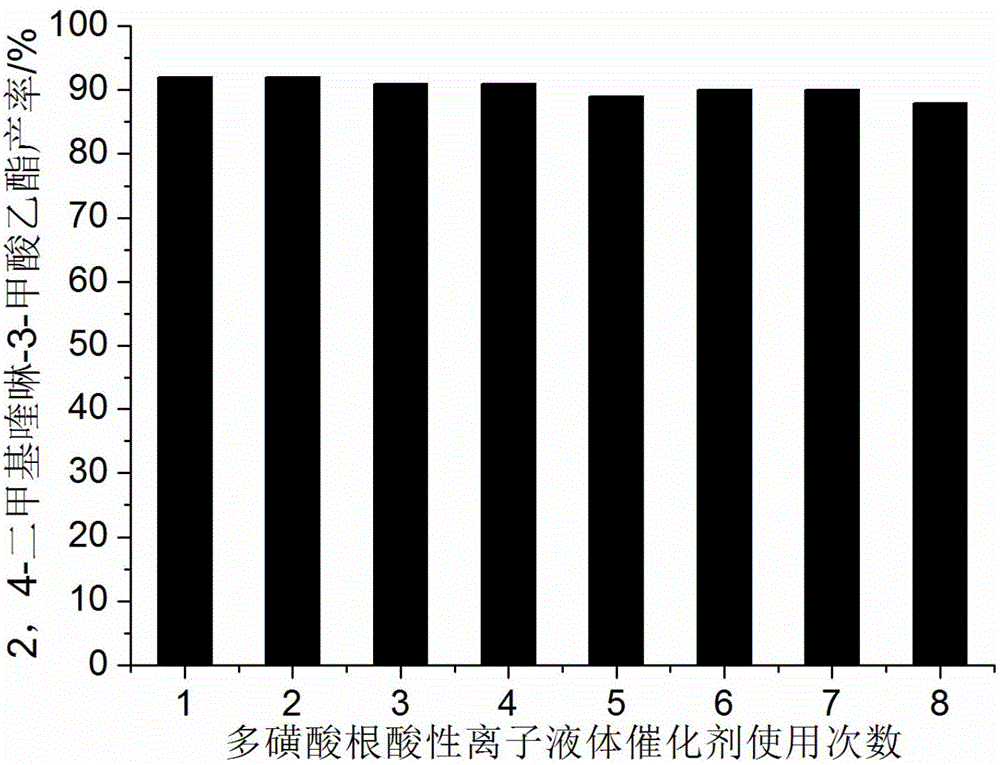
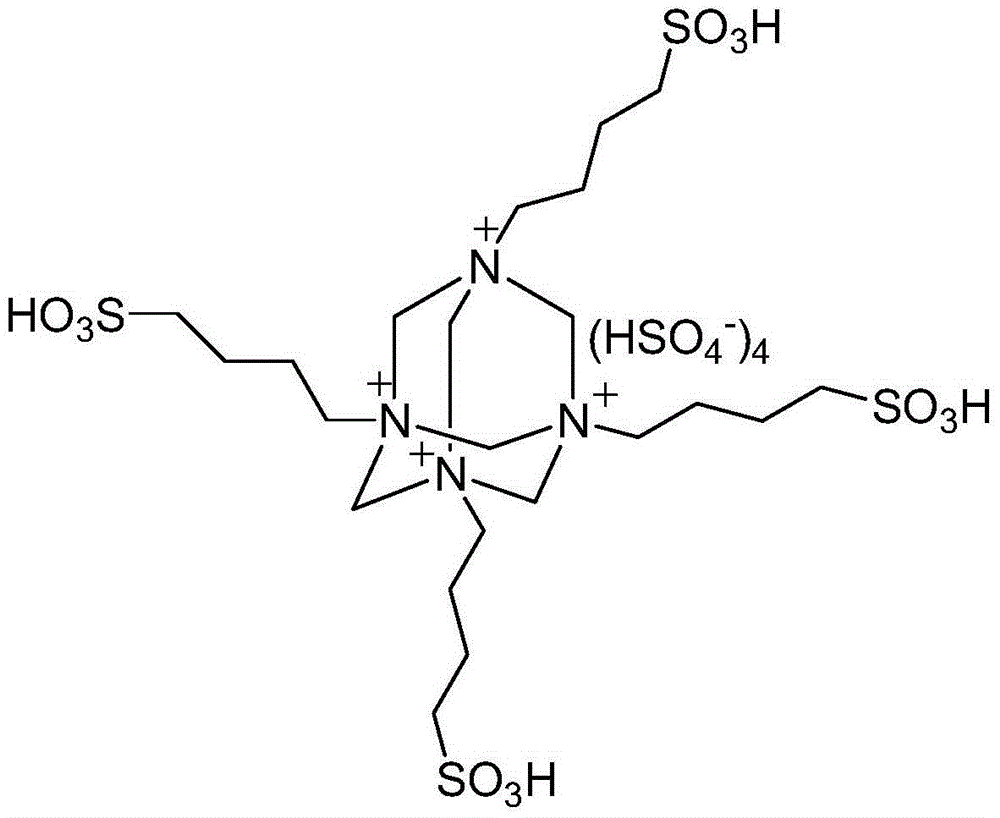
[0024] Add 10mmol 2-aminoacetophenone, 10mmol ethyl acetoacetate, 0.8mmol polysulfonate acidic ionic liquid catalyst and 30ml 75% ethanol aqueous solution into a 100ml single-necked bottle equipped with a condenser. The reaction was heated to reflux, and the progress of the reaction was tracked by thin-plate chromatography (TLC) (developing solvent: ethyl acetate:petroleum ether=1:4). The reaction took 7 minutes. After the reaction was completed, it was cooled to room temperature, filtered, and the resulting filter residue was vacuum-dried to obtain pure ethyl 2,4-dimethylquinoline-3-carboxylate with a yield of 92%. The filtrate was directly added with 2-aminoacetophenone and ethyl acetoacetate for repeated use.
[0025] 2,4-Dimethylquinoline-3-carboxylic acid ethyl ester: 1 H NMR (300MHz, CDCl 3 ): δ=1.62(t, J=7.0Hz, 3H), 2.97(s, 3H), 3.08(s, 3H), 4.69(q, J=7.0Hz, 2H), 7.72~8.33(m, 4H)
Embodiment 2
[0027] Add 10mmol 2-aminoacetophenone, 10mmol cyclopentanone, 0.8mmol polysulfonate acidic ionic liquid catalyst and 30ml 75% ethanol aqueous solution into a 100ml single-necked bottle equipped with a condenser. The reaction was heated to reflux, and the progress of the reaction was tracked by thin-plate chromatography (TLC) (developing solvent: ethyl acetate:petroleum ether=1:4). The reaction time was 6 minutes. After the reaction was completed, it was cooled to room temperature, filtered, and the resulting filter residue was vacuum-dried to obtain pure 9-methyl-2,3-dihydro-1H-cyclopenta[b]quinoline with a yield of 93%. Directly add 2-aminoacetophenone and cyclopentanone to the filtrate for repeated use.
[0028] 9-methyl-2,3-dihydro-1H-cyclopenta[b]quinoline: 1 H NMR (300MHz, CDCl 3 ): δ=2.05(m, 2H), 2.41(s, 3H), 2.88(s, J=7.5Hz, 2H), 3.23(t, J=7.0Hz, 2H), 7.39~7.94(m, 4H)
Embodiment 3
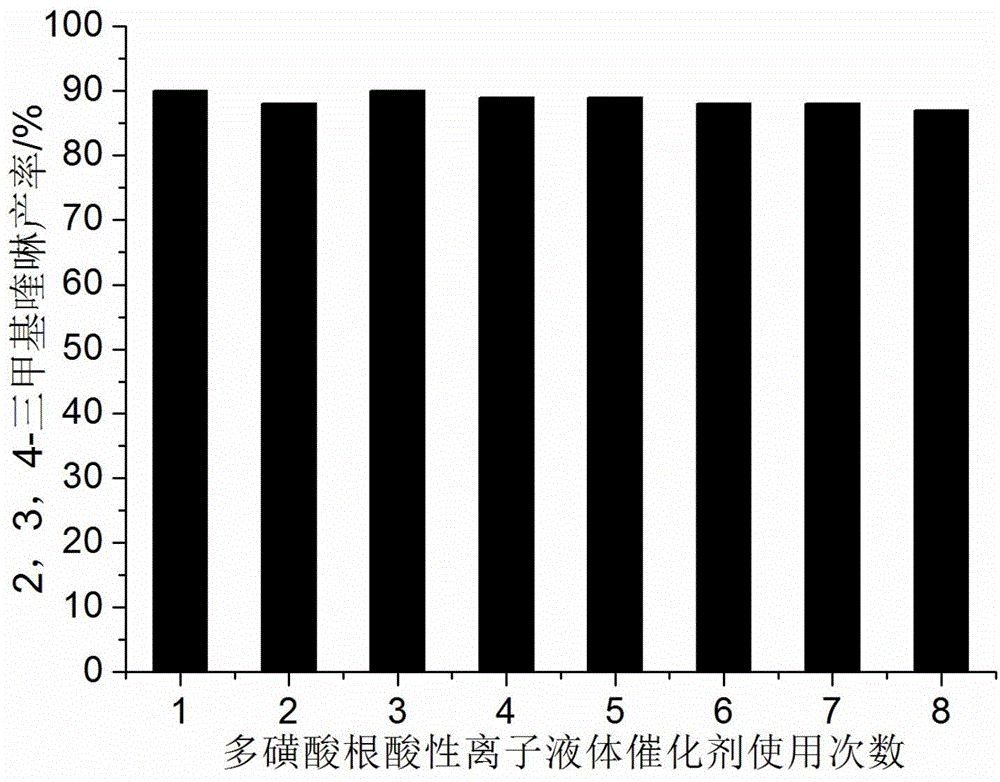
[0030] Add 10mmol 2-aminoacetophenone, 10mmol cyclohexanone, 0.7mmol polysulfonate acidic ionic liquid catalyst and 40ml 75% ethanol aqueous solution into a 100ml single-necked bottle equipped with a condenser. The reaction was heated to reflux, and the progress of the reaction was tracked by thin-plate chromatography (TLC) (developing solvent: ethyl acetate:petroleum ether=1:4). The reaction took 9 minutes. After the reaction was completed, it was cooled to room temperature, filtered, and the resulting filter residue was vacuum-dried to obtain pure 9-methyl-1,2,3,4-tetrahydroacridine with a yield of 95%. Directly add 2-aminoacetophenone and cyclohexanone to the filtrate for repeated use.
[0031] 9-methyl-1,2,3,4-tetrahydroacridine: 1 H NMR (300MHz, CDCl 3): δ=1.69(m, 4H), 2.21(s, 3H), 2.58(t, J=7.6Hz, 2H), 2.89(t, J=7.6Hz, 2H), 7.17~7.80(m, 4H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com