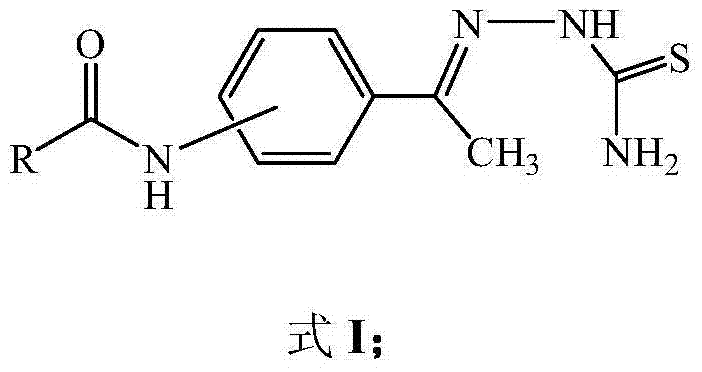
Aminoacetophenone thiosemicarbazone derivative and application thereof
A technology of aminoacetophenone thiosemicarbazide and derivatives, which is applied in the fields of cosmetics, medicines, food chemistry and agricultural pesticides, can solve the problems of insufficient safety and poor activity, and achieves simple preparation and strong inhibitory activity. , the effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
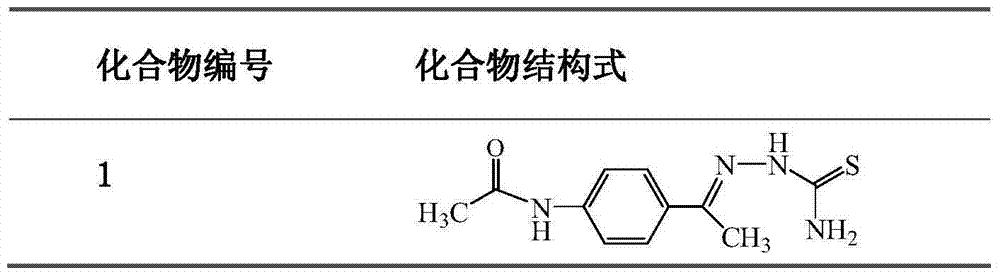
[0024] Embodiment 1: the synthesis of 4'-acetamidoacetophenone thiosemicarbazone (compound 1):
[0025] Step 1, 4-aminoacetophenone obtains amidated acetophenone through carboxylic acid amidation reaction (this step is prior art): add p-aminoacetophenone (0.02mol), carboxylic acid (0.08 mol) and 1-2 grains of zeolite, the solution was heated slowly to keep the reactants slightly boiling for about 15 minutes. Then gradually increase the temperature to 100-105°C for about 45 minutes. When the thermometer reading drops, stop heating. Under constant stirring, the reactant was slowly poured into a beaker filled with 100ml of cold water while hot, continued to stir, fully cooled, and the amidated acetophenone crude product was separated out. Suction filter and wash the crude product with 5-10ml of cold water. Then washed 3 times with 20 mL of hot water, dried and recrystallized with a small amount of ethanol to obtain the product amidated acetophenone.
[0026] Step 2, the amida...
Embodiment 2
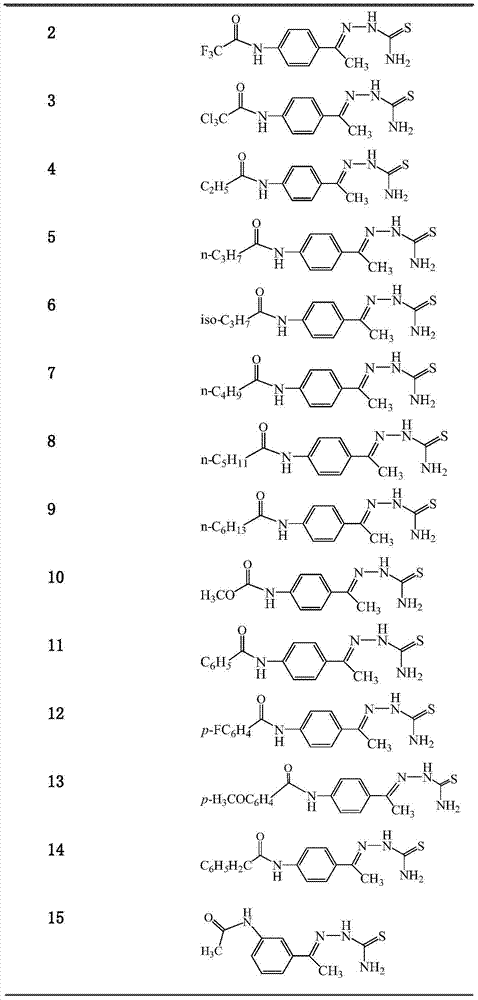
[0027] Embodiment 2: the synthesis of 4'-trifluoroacetamidoacetophenone thiosemicarbazone (compound 2)
[0028] Referring to Example 1 for the synthetic method, the obtained target product (compound 2) was a colorless solid with a yield of 92%. 1 H NMR (300MHz, DMSO-d 6 ):δ11.37(s,1H),10.26(s,1H),8.33(s,1H),8.02(d,J=8.7Hz,3H),7.72(d,J=8.7Hz,2H),2.31 (s,3H). 13 C NMR (75MHz, DMSO-d 6 ): δ178.8, 154.7, 154.2, 146.9, 137.1, 134.6, 127.3, 120.4, 13.7. ESI-MS (m / z) 305.1 [M+1] + .
Embodiment 3
[0029] Embodiment 3: the synthesis of 4'-trichloroacetamidoacetophenone thiosemicarbazone (compound 3)
[0030] Referring to Example 1 for the synthetic method, the obtained target product (compound 3) was a light yellow solid with a yield of 80%. 1 H NMR (300MHz, DMSO-d 6 ):δ10.95(s,1H),10.23(s,1H),8.30(s,1H),8.00(d,J=8.8Hz,3H),7.70(d,J=8.8Hz,2H),2.29 (s,3H). 13 C NMR (75MHz, DMSO-d 6 ): δ178.8, 159.6, 147.0, 138.0, 134.3, 127.2, 120.6, 92.9, 13.7. ESI-MS (m / z) 353.2 [M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com