3, 3-diazido methyl oxetane-ethylene glycol energetic copolyether with alternating multi-block structure and synthesis method thereof
A kind of technology of bis-azidomethyl oxetane and synthesis method, applied in 3 fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1.05g of PBAMO (Mn=301, 3.5mmol) was dissolved in 10mL of THF, 2.24g of KOH (40mmol) was added, and the system was transferred to a constant temperature oil bath at 65°C. A THF solution of 1.12 g of tosylate-terminated polyethylene glycol (Mn=510, 2.2 mmol) was slowly dropped into the above reaction system, and the system continued to react at 65° C. for 24 h after the addition was completed. Then filter and rotary evaporate, the crude product was dissolved in dichloromethane, washed with distilled water until neutral. Then dried with anhydrous magnesium sulfate, suction filtered, and rotary evaporated, followed by adding petroleum ether with a boiling point of 60-90°C and methanol to wash and rotary evaporated to obtain a yellow sticky substance (0.78g)
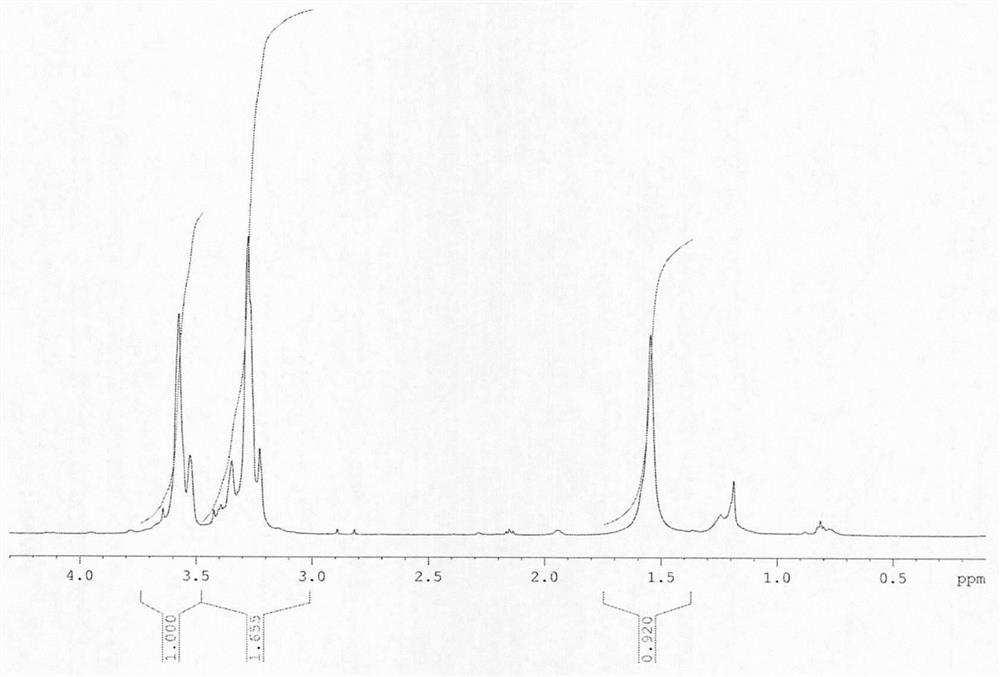
[0024] Structure Identification:
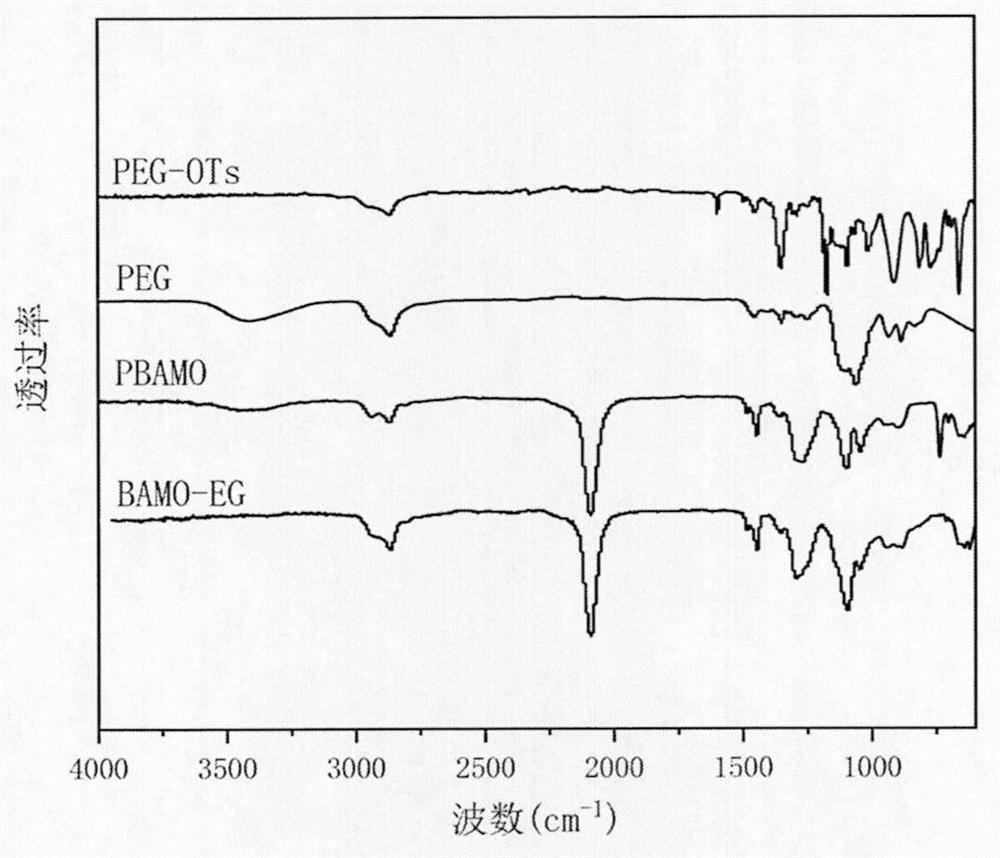
[0025] FT-IR infrared: After PEG-OTS undergoes p-tosylation to obtain terminal tosylate polyethylene glycol, the infrared hydroxyl 3000-3500cm -1 disappear, proving that the end gr...
Embodiment 2
[0029] Dissolve 0.93g of PBAMO (Mn=301, 3.1mmol) in 20mL of THF, add 2.32g of KOH (41mmol), and transfer the system into a 65°C constant temperature oil bath. A THF solution of 1..01 g of tosylate-terminated glycerol (Mn=460, 2.2 mmol) was slowly dropped into the above reaction system, and the system continued to react at 65° C. for 24 h after the addition was completed. Then filter and rotary evaporate, the crude product was dissolved in dichloromethane, washed with distilled water until neutral. It was then dried over anhydrous magnesium sulfate, suction filtered, and rotary evaporated, followed by adding petroleum ether with a boiling point of 60-90°C and methanol for washing and rotary evaporation to obtain a yellow viscous substance (0.65 g).
Embodiment 3
[0031] Dissolve 0.95g of PBAMO (Mn=367, 2.6mmol) in 20mL of THF, add 2.01g of KOH (36mmol), and transfer the system into a 65°C constant temperature oil bath. A THF solution of 0.83 g of tosylate-terminated glycerol (Mn=460, 1.8 mmol) was slowly dropped into the above reaction system, and the system continued to react at 65° C. for 24 h after the addition was completed. Then filter and rotary evaporate, the crude product was dissolved in dichloromethane, washed with distilled water until neutral. It was then dried over anhydrous magnesium sulfate, suction filtered, and rotary evaporated, followed by adding petroleum ether with a boiling point of 60-90°C and methanol to wash and rotary evaporated to obtain a yellow sticky substance (0.52 g).
[0032] figure 1 The BAMO-EG alternating multi-block copolymer physical figure prepared for embodiment 1;
[0033] figure 2 For the Fourier transform infrared characteristic spectrogram of the BAMO-EG alternating multi-block copolymer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com