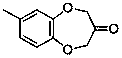
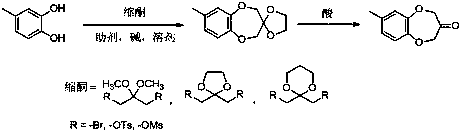
Watermelon ketone preparation method
A watermelon ketone and acetone technology, which is applied in the field of preparation of flavor and fragrance compounds, can solve the problems of poor chlorinated acetone activity, low phenolic hydroxyl condensation efficiency, increased by-products and the like, and achieves easy purification, easy reaction and reduced side reactions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Into a 1000 mL three-neck flask equipped with a thermometer, reflux condenser and constant pressure dropping funnel, add 210 mL of ethanol and 90 mL of water, 60 g of potassium hydroxide, and 5 g of potassium iodide, and place under vigorous magnetic force at 1000 r / min. Stir and slowly add 62 g of 4-methylcatechol and 130 g of ethanol solution of 1,3-dibromoacetone ketal, which are mixed evenly, dropwise under reflux, and continue to reflux for 4 h after the dropwise addition is complete. After the reaction is complete, stop stirring, cool, filter with suction, and recover the solvent by rotary evaporation. The remaining dark brown oil is dissolved in dichloromethane and washed with 5% sodium hydroxide solution, saturated sodium carbonate solution and saturated sodium chloride solution successively. until colorless, dried, spin-dried, and distilled under reduced pressure to obtain 97.9 g of a ketal product with a purity of 98%, with a yield of 90%.
Embodiment 2
[0027] In a 1000 mL three-necked flask equipped with a thermometer, a reflux condenser and a constant pressure dropping funnel, add 300 mL of acetone, 60 g of potassium hydroxide, and 5 g of ammonium iodide, and stir vigorously at 1000 r / min. , under reflux state, slowly add dropwise acetone solution of 62 g of 4-methylcatechol and 130 g of 1,3-dibromoacetone ethylene acetal, which are uniformly mixed, and continue to reflux for 4 h after the dropwise addition is completed. After the reaction is complete, stop stirring, cool, filter with suction, and recover the solvent by rotary evaporation. The remaining dark brown oil is dissolved in dichloromethane and washed with 5% sodium hydroxide solution, saturated sodium carbonate solution and saturated sodium chloride solution successively. until colorless, dried, spin-dried, and distilled under reduced pressure to obtain 99.1 g of a ketal product with a purity of 98%, with a yield of 91%.
Embodiment 3
[0029] Into a 1000 mL three-neck flask equipped with a thermometer, reflux condenser and constant pressure dropping funnel, add 210 mL of ethanol and 90 mL of water, 60 g of potassium hydroxide, and 5 g of potassium iodide, and place under vigorous magnetic force at 1000 r / min. Stir, slowly add dropwise the ethanol solution of 62 g of 4-methylcatechol and 221 g of 1,3-di-p-toluenesulfonate acetone ketal mixed uniformly under the reflux state, and continue to Reflux for 4 h. After the reaction is complete, stop stirring, cool, filter with suction, and recover the solvent by rotary evaporation. The remaining dark brown oil is dissolved in dichloromethane and washed with 5% sodium hydroxide solution, saturated sodium carbonate solution and saturated sodium chloride solution successively. until colorless, dried, spin-dried, and distilled under reduced pressure to obtain 102.3 g of a ketal product with a purity of 98%, and a yield of 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com