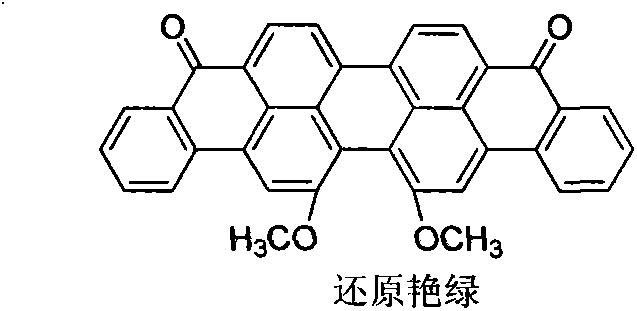
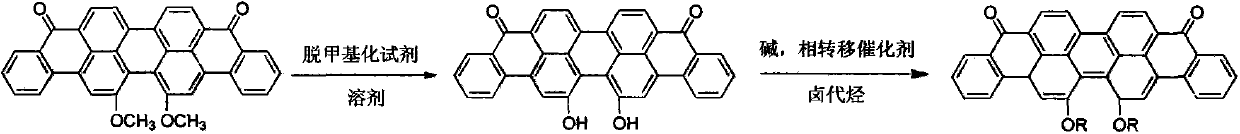
Method for preparing 16,17-dialkoxyviolanthrone derivatives
A technology of bis-alkoxy anthrone and dimethoxy anthrone violet, which is applied in 16 fields, can solve the problems of low reaction yield of demethylation reagents and complex products, and achieve short reaction routes, high yields, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 16
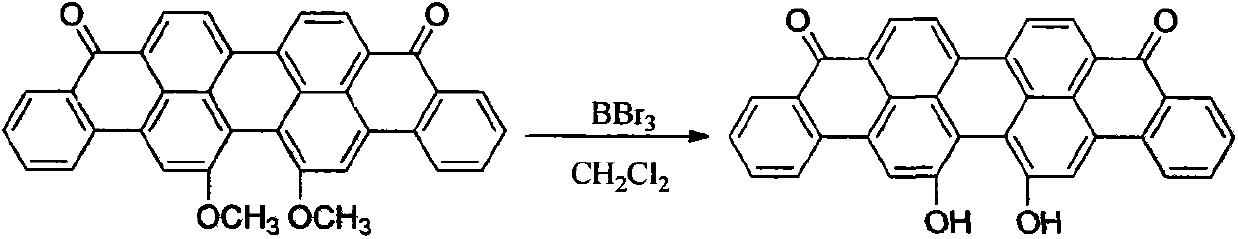
[0029] Embodiment one, the synthesis of 16,17-dihydroxyanthrone violet
[0030] The synthesis of 16,17-dihydroxyanthrone violet uses 16,17-dimethoxyanthrone violet as raw material and dichloromethane as solvent BBr 3 As a demethylation reagent, the target compound is obtained.
[0031]
[0032] Add 8.03g (15.5mmol) of 16,17-dimethoxyanthrone violet and 100mL of dichloromethane into a 250mL round-bottomed flask, and stir at room temperature for 1 hour to fully disperse them. Under the condition of stirring, under the condition of ice-water bath, 7.80 g (31 mmol) of boron tribromide were added. After the dropwise addition was completed, the mixture was fully stirred at room temperature for 8 hours. The reaction was further heated to reflux for 8 hours. According to TLC tracking, the raw material was almost completely reacted, and the reaction was stopped. After the system temperature dropped to room temperature. The reaction solution was poured into 500 mL of about 2 mol...
Embodiment 2 16
[0034] Embodiment two, 16, the synthesis of 17- didecyloxy anthrone violet
[0035]
[0036] Take 1.98g (4.10mmol) of 16,17-dihydroxyanthrone violet into a 100mL round bottom flask, then add 20mL of N,N-dimethylformamide, stir and raise the temperature to 80°C to fully disperse into the reaction system , add 1.60g (11.5mmol) anhydrous K 2 CO 3 , and then 1.81 g (8.2 mmol) of 1-bromodecane, and then 0.1 g of 18-crown-6 as a catalyst, and continued the reaction at 80° C. for 5 hours. Filter while hot to remove insoluble impurities and inorganic salts. Under the condition of stirring, the reaction solution was added into 100mL water, and the product was precipitated. Filter with suction and dry to obtain a blue-black powdery solid. Recrystallization from ethyl acetate gave 16,17-didecyloxyanthrone violet. After drying, 2.36 g of product was obtained, the yield was 74%.
Embodiment 3 16
[0037] Embodiment three, preparation of 16,17-dioctyloxyanthrone violet
[0038] Take 1.21g (2.48mmol) of 16,17-dihydroxyanthrone violet in a 100mL flask, add 50mL of N-methylpyrrolidone, and heat for 10min to 100°C to disperse 16,17-dihydroxyanthrone violet as much as possible. Then add 1.00g (7.2mmol) of anhydrous K 2 CO 3 , 0.98 g (5.08 mmol) of 1-bromooctane, and a catalytic amount of 18-crown-6. Keep the reaction temperature at 100° C., and stop the reaction for 8 hours.
[0039] Pour the reaction solution into 100 mL of water, stir well, filter, and dry to obtain the crude product. The crude product was dissolved in dichloromethane, and the insoluble components were removed by filtration. The dichloromethane was spin-dried to obtain a solid, and after drying, 1.26 g of the product was obtained, and the yield was 72%. through a silica gel chromatographic column, with CH 2 Cl 2 :CH 3 OH=20:1 is used as the eluent for further elution, separation and purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com