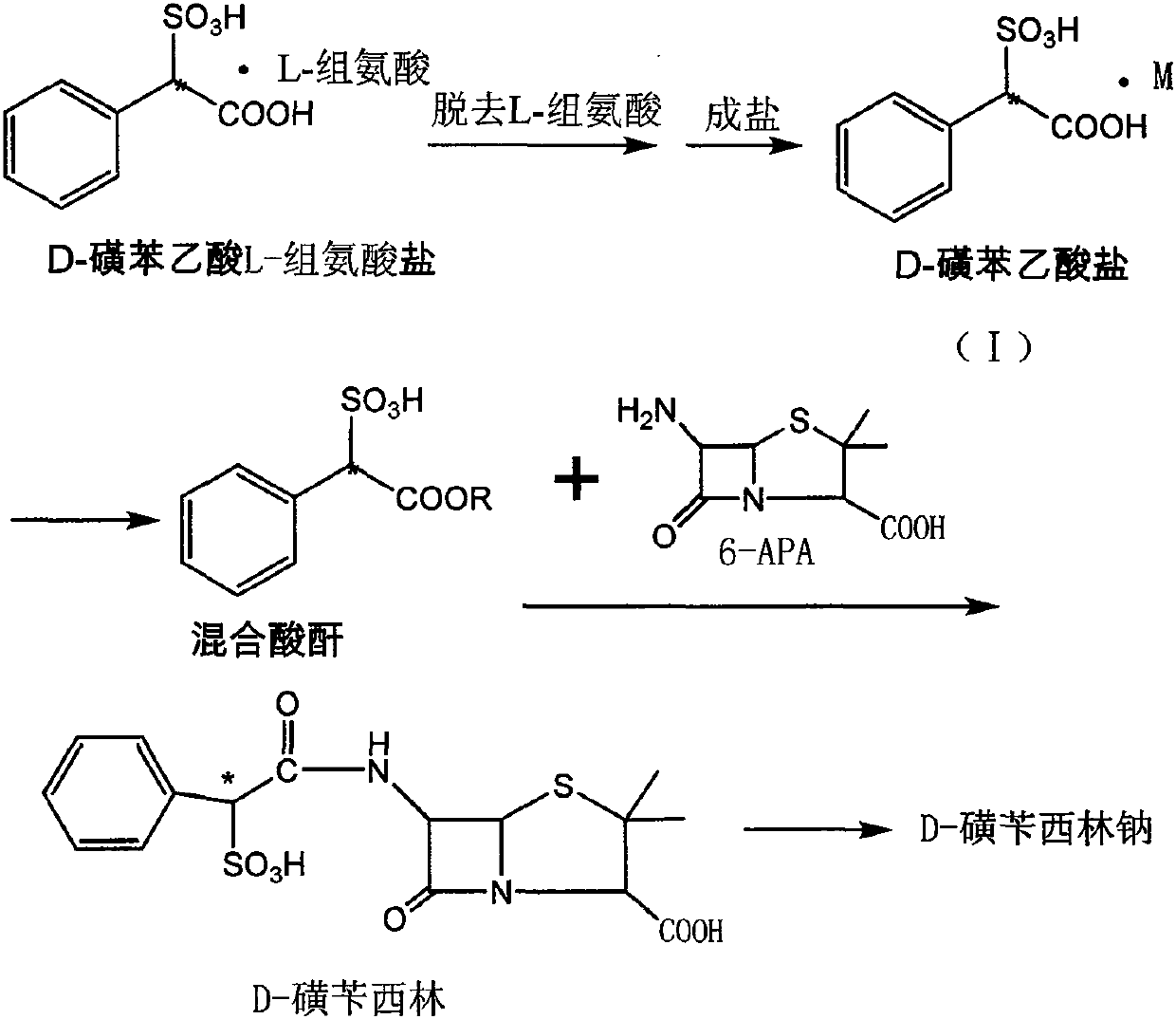
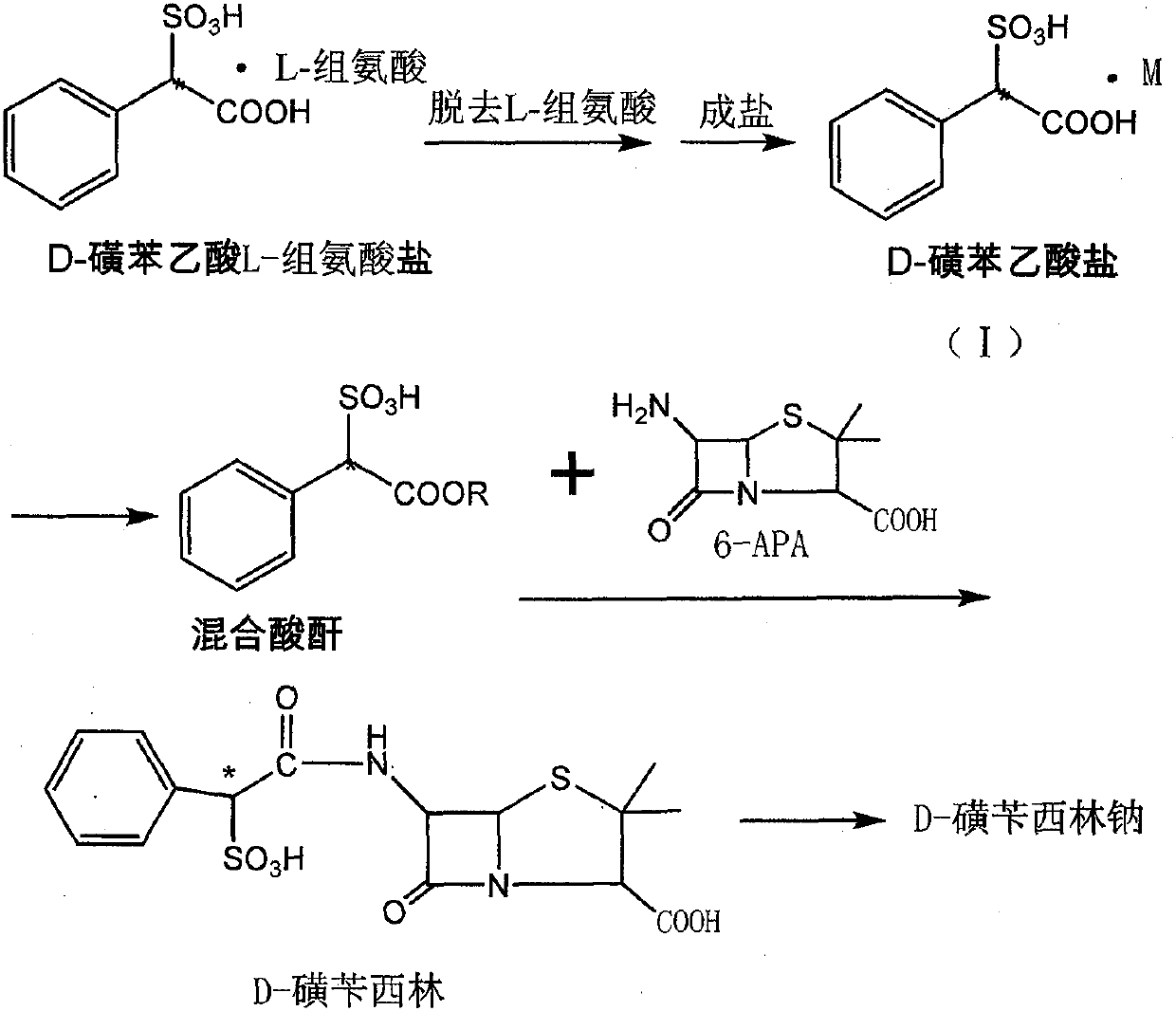
A kind of preparation method of d-sulbenicillin sodium
A technology of sulfobenicillin sodium and sulfophenylacetic acid, applied in the field of pharmaceutical synthesis, can solve the problems such as the unmentioned preparation method of D-sulfophenylacetic acid triethylamine salt, unstable intermediates, non-separation of acid chlorides, etc., and achieve process stability Feasible, high product yield, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 D-sulfophenylacetic acid sodium salt
[0030] 100 g of D-sulfophenylacetic acid L-histidine salt (0.270 mol) was suspended in a mixed solvent of 1000 ml of acetone and 100 ml of ethanol. Add triethylamine, stir for 30 minutes, filter and recover L-histidine, add sodium isooctanoate, and stir for 1-2 hours. The solid was collected by filtration and dried under reduced pressure to obtain 45.6 g of sodium D-sulfophenylacetate.
Embodiment 2
[0031] The preparation of embodiment 2 D-sulfophenylacetic acid potassium salt
[0032] As in Example 1, potassium isooctanoate was used to replace sodium isooctanoate to obtain 47.2 grams of potassium salt of D-sulfophenylacetic acid.
Embodiment 3
[0033] The preparation of embodiment 3 D-sulfophenylacetic acid triethylamine salt
[0034] 100 g of D-sulfophenylacetic acid L-histidine salt (0.270 mol) was suspended in a mixed solvent of 1000 ml of acetone and 100 ml of ethanol. Add triethylamine, stir for 30 minutes, and filter to recover L-histidine. Add 13.0 g of formic acid. Stir for 1-2h. The solid was collected by filtration and dried under reduced pressure to obtain 76.3 g of D-sulfophenylacetic acid triethylamine salt.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com