A kind of preparation method of rivaroxaban intermediate and new synthesis method of rivaroxaban
A compound and reaction time technology, applied in the direction of organic chemistry, can solve the problems of unstable toxicity, low optical purity, product racemization, etc., and achieve the effect of high safety, high optical purity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the preparation of (S)-N-glycidyl phthalimide
[0054] I. Synthesis of (S)-N-2,3-dihydroxypropylphthalimide
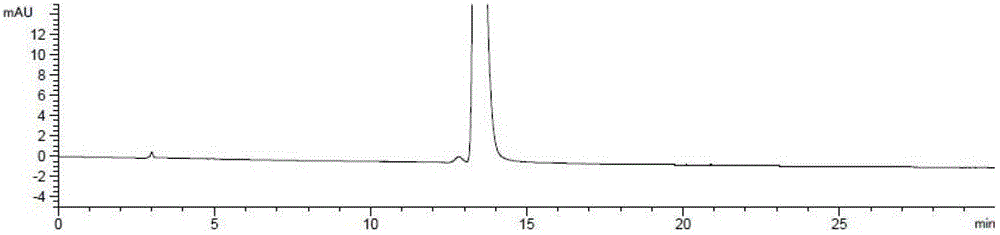
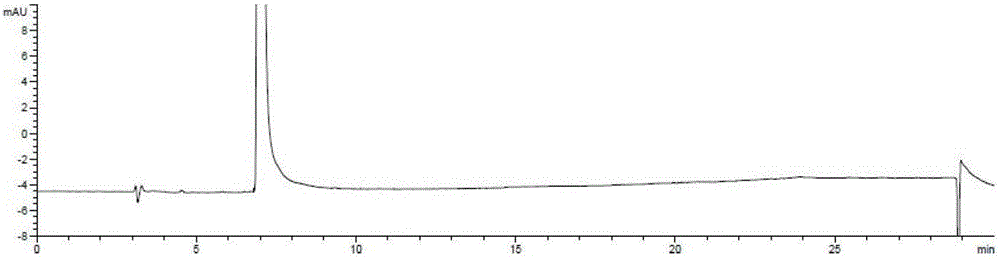
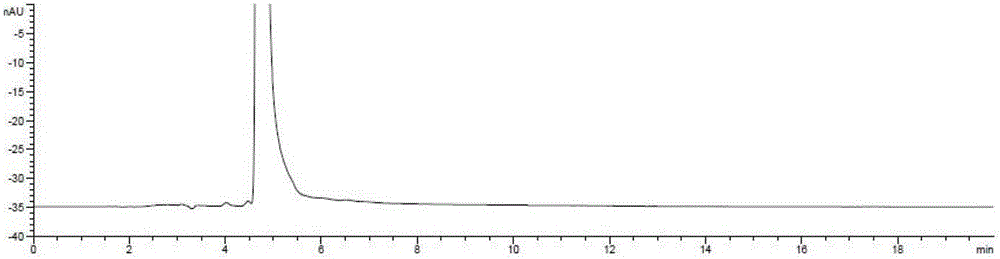
[0055] Under nitrogen protection, in a 250ml reaction flask, (R)-3-chloro-1,2-propanediol (5.0g, 45.2mmol) and phthalimide potassium salt (10.0g, 54.0mmol) were mixed in In 100ml of absolute ethanol, the temperature was raised to 78°C, and the reaction was refluxed for 12 hours. After completion of the reaction, filter, and concentrate the filtrate to dryness under reduced pressure, add 100ml of ethyl acetate and 100ml of water, extract and separate layers, wash the organic layer with 50ml of saturated brine, and concentrate to dryness under reduced pressure to obtain (S)-N-2, 3-Dihydroxypropylphthalimide (9.0 g), yield: 90%, optical purity: 99.5%.
[0056] II. Preparation of (S)-N-glycidylphthalimide
[0057] Under nitrogen protection, in a 250ml reaction flask, (S)-N-2,3-dihydroxypropylphthalimide (5.0g, 22.6mmol) prepared in step I was mixed wi...
Embodiment 2
[0066] Embodiment 2: the preparation of (S)-N-glycidyl phthalimide
[0067] I. Synthesis of (S)-N-2,3-dihydroxypropylphthalimide
[0068] Under nitrogen protection, in a 250ml reaction flask, (R)-3-bromo-1,2-propanediol (5.0g, 32.3mmol) and phthalimide potassium salt (9g, 48.6mmol) were dissolved in 100ml The temperature was raised to 78° C. in absolute ethanol, and the reaction was refluxed for 14 hours. After the reaction was completed, the filtrate was concentrated to dryness under reduced pressure, 100ml of ethyl acetate and 100ml of water were added, the layers were extracted, the organic layer was washed with 50ml of saturated brine, and then concentrated to dryness under reduced pressure to obtain (S)-N-2,3 - Dihydroxypropylphthalimide (6.6 g), yield: 92%, optical purity: 99.5%.
[0069] II. Preparation of (S)-N-glycidylphthalimide
[0070] Under nitrogen protection, in a 250ml reaction flask, the (S)-N-2,3-dihydroxypropylphthalimide (5.0g, 22.6mmol) obtained in step...
Embodiment 3
[0071] Embodiment 3: the preparation of (S)-N-glycidyl phthalimide
[0072] I. Synthesis of (S)-N-2,3-dihydroxypropylphthalimide
[0073] Under nitrogen protection, in a 250ml reaction flask, (R)-3-iodo-1,2-propanediol (5.0g, 24.8mmol) and phthalimide sodium salt (4.2g, 24.8mmol) were mixed in Heat up to 82°C in 100ml of isopropanol, and reflux for 8 hours. After the reaction was completed, the filtrate was concentrated to dryness under reduced pressure, 100ml of ethyl acetate and 100ml of water were added, the layers were extracted, the organic layer was washed with 50ml of saturated brine, and then concentrated to dryness under reduced pressure to obtain (S)-N-2,3 - Dihydroxypropylphthalimide (4.9 g), yield: 89%, optical purity: 99.4%.
[0074] II. Preparation of (S)-N-glycidylphthalimide
[0075] Under nitrogen protection, in a 250ml reaction flask, the (S)-N-2,3-dihydroxypropylphthalimide (4.5g, 20mmol) prepared in step I and pyridine (3.5g, 44mmol ) was dissolved in 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com