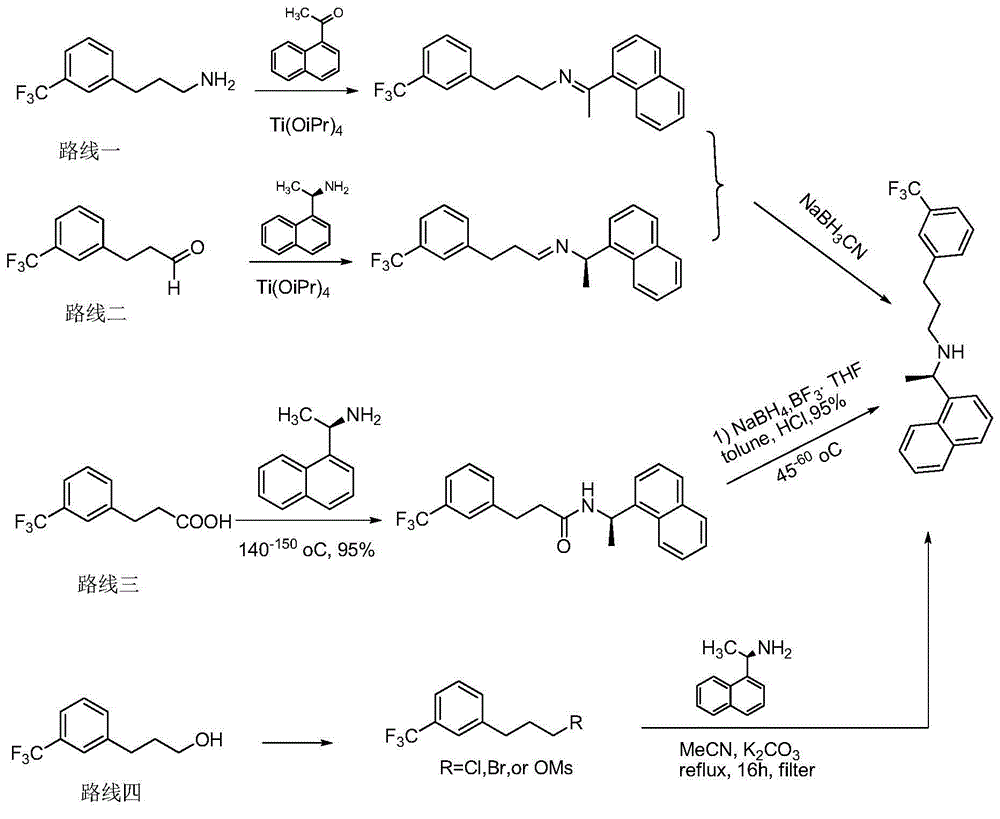
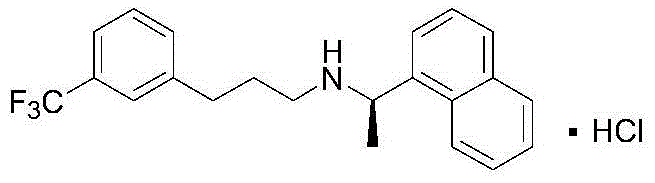
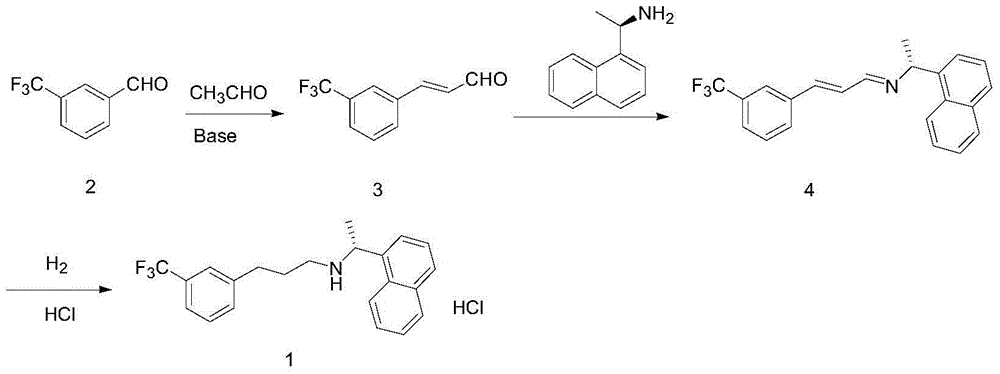
Synthesis method of cinacalcet
A synthetic method and technology of lipase, which are applied in the field of new preparation routes of cinacalcet, can solve the problems of difficult preparation of m-trifluoromethamphetamine, low optical purity of products, inconvenience of boron trifluoride, etc., and achieve optical purity Guaranteed, good racemization effect, and the effect of improving the optical purity of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]1) Preparation of (R)-1-(1-naphthyl)ethylamine: In a 250mL three-neck flask, mechanically stir at a slow speed, add 40mL toluene, 4 g of racemic naphthylethylamine, acyl donor p-chlorophenol valeric acid Esters 5.2g, 1g Pd / LDH-SA and 0.4g lipase Novozym 435, the hydrogen pressure is 0.01MPa, react 15h under the condition of 55 ℃, detect by gas chromatography, after the reaction of naphthylethylamine is complete, remove the catalyst (lipase and spin catalyst), for the next reaction, the toluene solution was washed 2 times with a solution of sodium hydroxide to remove p-chlorophenol, then dried and evaporated to dryness under reduced pressure to obtain (R)-amide; the obtained (R) - Amide was hydrolyzed in 2mol / L dilute hydrochloric acid at 80°C for 10h, and then ammonia water was added to adjust the pH>10. After 0.5h, methylene chloride was extracted, dried, and rotary evaporated to obtain 3.53g (R)-1-(1-naphthyl) Ethylamine, the yield is 88.3%.
[0041] 2) Synthesis of c...
Embodiment 2
[0052] 1) Preparation of (R)-1-(1-naphthyl)ethylamine: In a 250mL three-neck flask, mechanically stir at a slow speed, add 40mL toluene, 4 g of racemic naphthylethylamine, acyl donor p-chlorophenol acetic acid 4.2g of ester, 1g of Pd / LDH-SA and 0.4g of lipase Novozym 435, the hydrogen pressure is 0.01MPa, reacted at 55°C for 15h, detected by gas chromatography, after the reaction of naphthylethylamine was complete, the catalyst (lipase and spin catalyst) for the next reaction, add 40ml of 2mol / L dilute hydrochloric acid to the toluene solution, heat up to 80°C, hydrolyze for 10h, cool, separate layers, adjust the pH of the aqueous layer to >10 with ammonia water, and dichloromethane after 0.5h After extraction, drying and rotary evaporation, 3.7 g of (R)-1-(1-naphthyl)ethylamine was obtained with a yield of 92.5%.
[0053] 2) Synthesis of cinacalcet:
[0054] (1) In a 250ml three-necked flask, add 120mL of KOH aqueous solution (1%), 20mL of ethanol, and 5g of m-trifluoromethy...
Embodiment 3
[0058] 1) Preparation of (R)-1-(1-naphthyl)ethylamine: In a 250mL three-neck flask, mechanically stir slowly, add 40mL of toluene, 4g of racemic naphthylethylamine, acyl donor p-chlorophenol propane Ester 4.5g, 1g Pd / LDH-SA and 0.4g lipase Novozym 435, hydrogen pressure is 0.01MPa, reacted 15h under the condition of 55 ℃, gas chromatography detects, after naphthylethylamine reacts completely, remove catalyst (lipase and racemization catalyst) for the next reaction, add 40ml of 2mol / L dilute hydrochloric acid to the toluene solution, heat up to 80°C, hydrolyze for 10h, cool, separate layers, adjust the pH of the aqueous layer to >10 with ammonia water, and dichloride after 0.5h After extraction with methane, drying and rotary evaporation, 3.7 g of (R)-1-(1-naphthyl)ethylamine was obtained, with a yield of 92.5%.
[0059] 2) Synthesis of cinacalcet: as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com