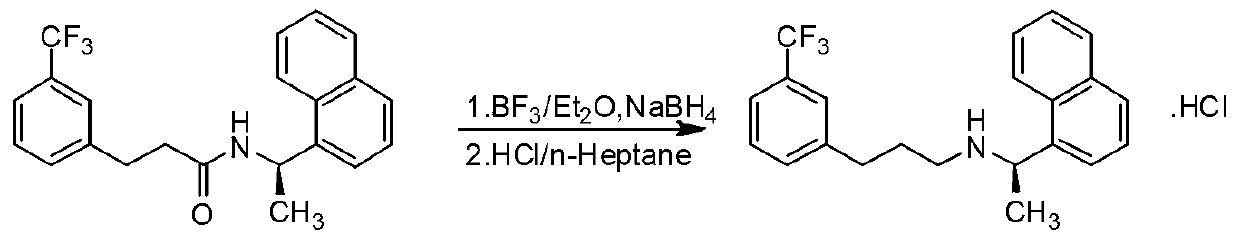Patents
Literature
116 results about "Cinacalcet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
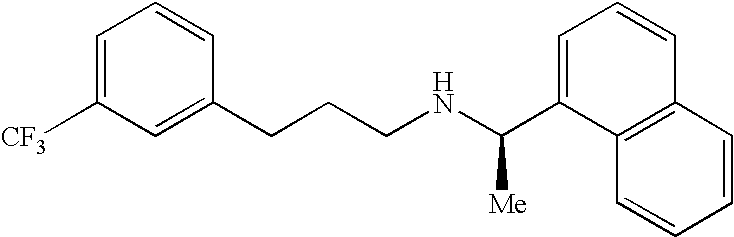
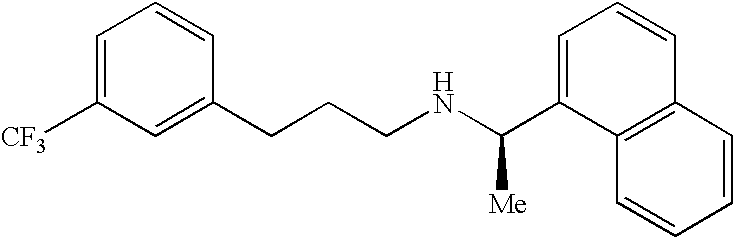
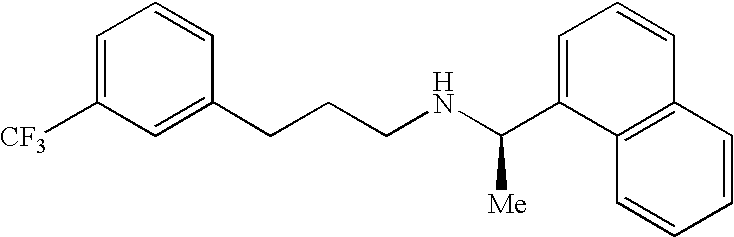
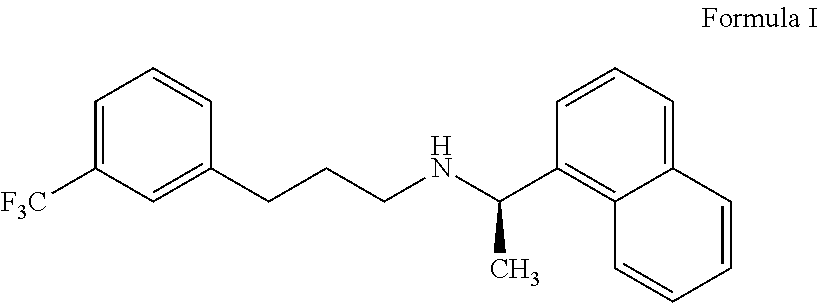
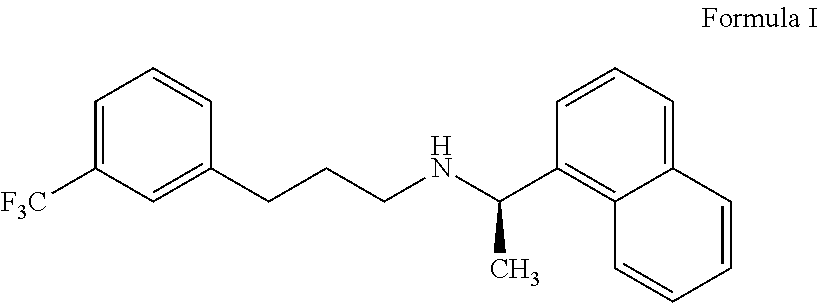
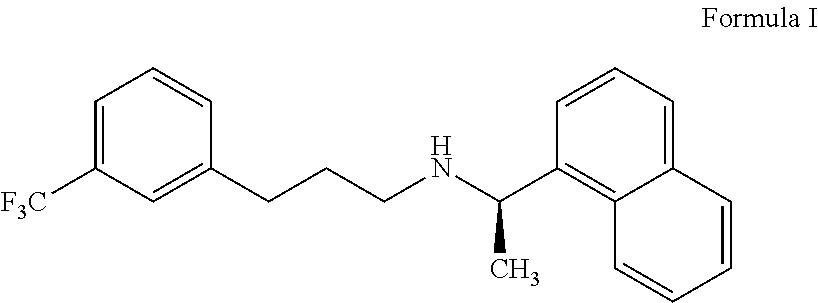
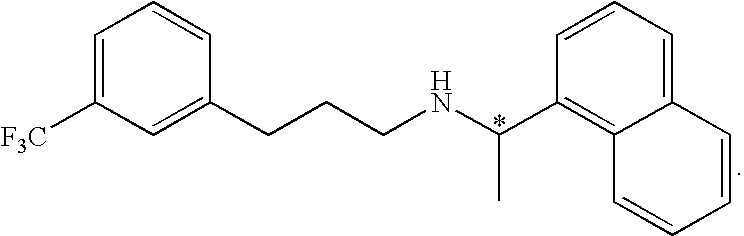
Cinacalcet is used to treat increased amounts of a certain hormone (parathyroid) in people with long-term kidney disease who are on dialysis. It is also used to treat increased amounts of calcium in people with an overactive parathyroid gland or in people with cancer of the parathyroid gland.
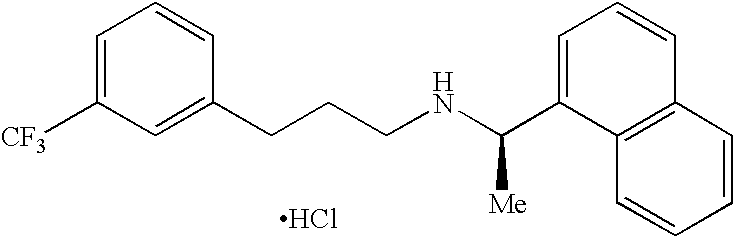
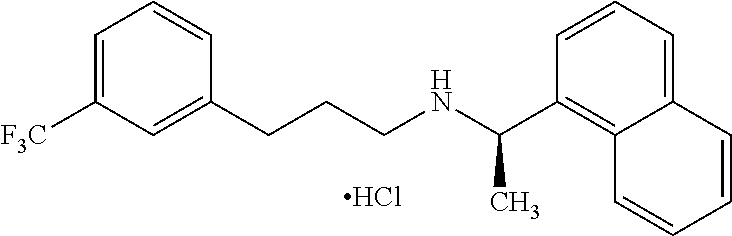
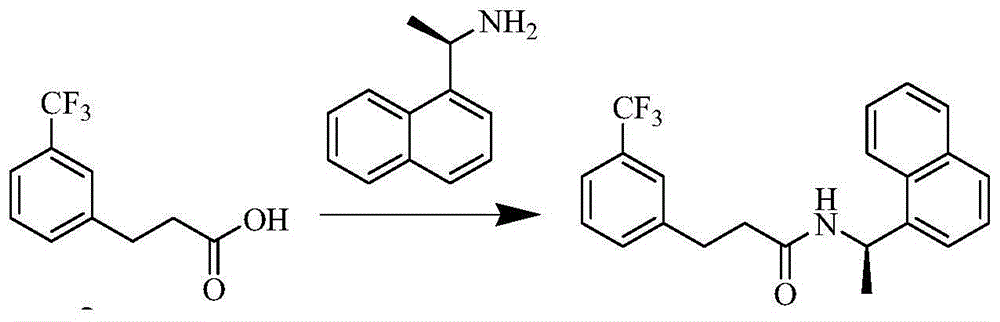
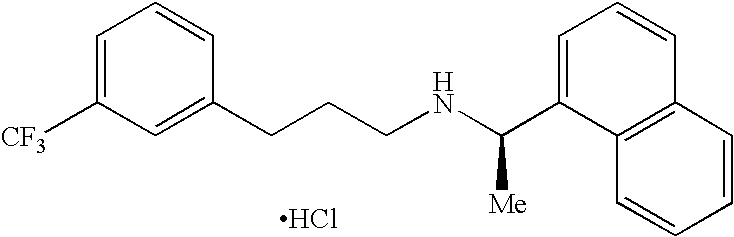
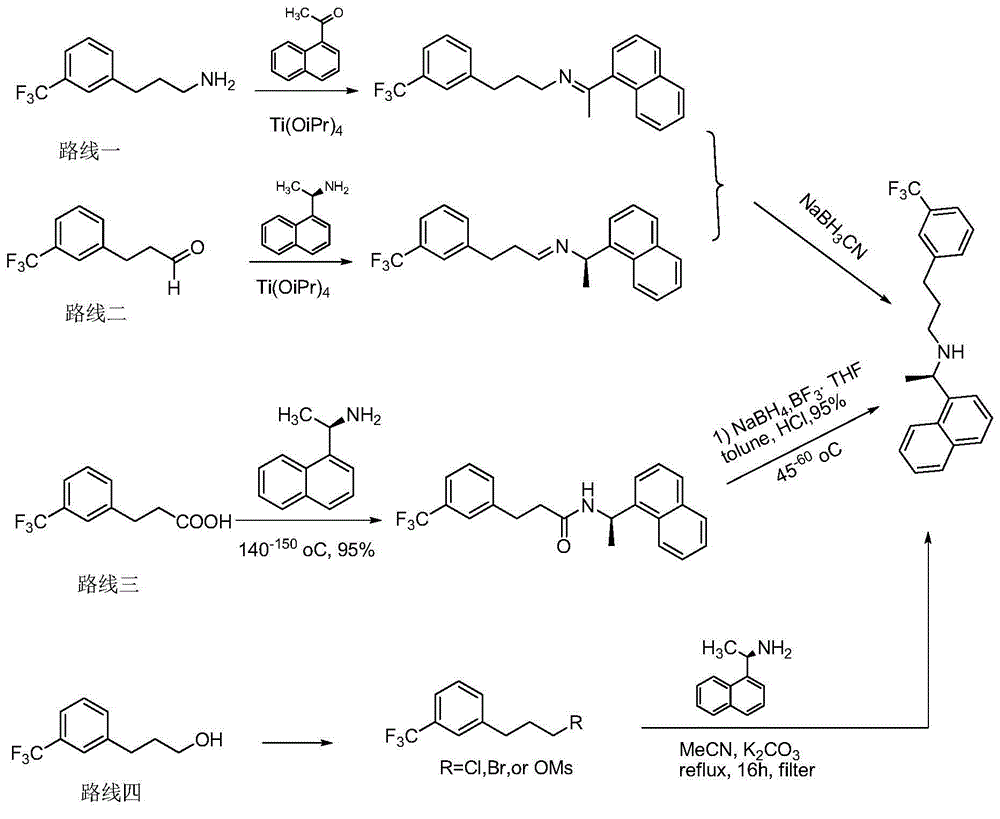
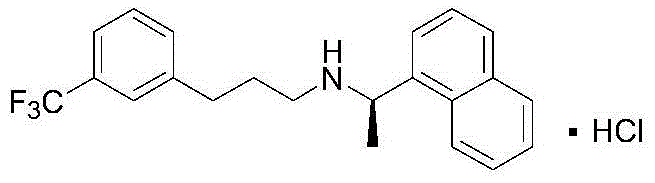
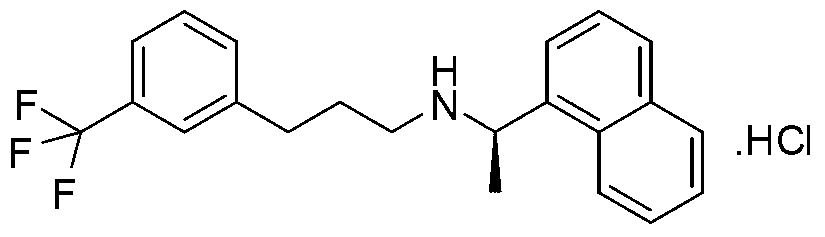
Process for preparing Cinacalcet hydrochloride
InactiveUS7250533B2Organic compound preparationOrganic chemistry methodsCinacalcetCinacalcet Hydrochloride
Owner:TEVA PHARM USA INC
Process for the preparation of cinacalcet base
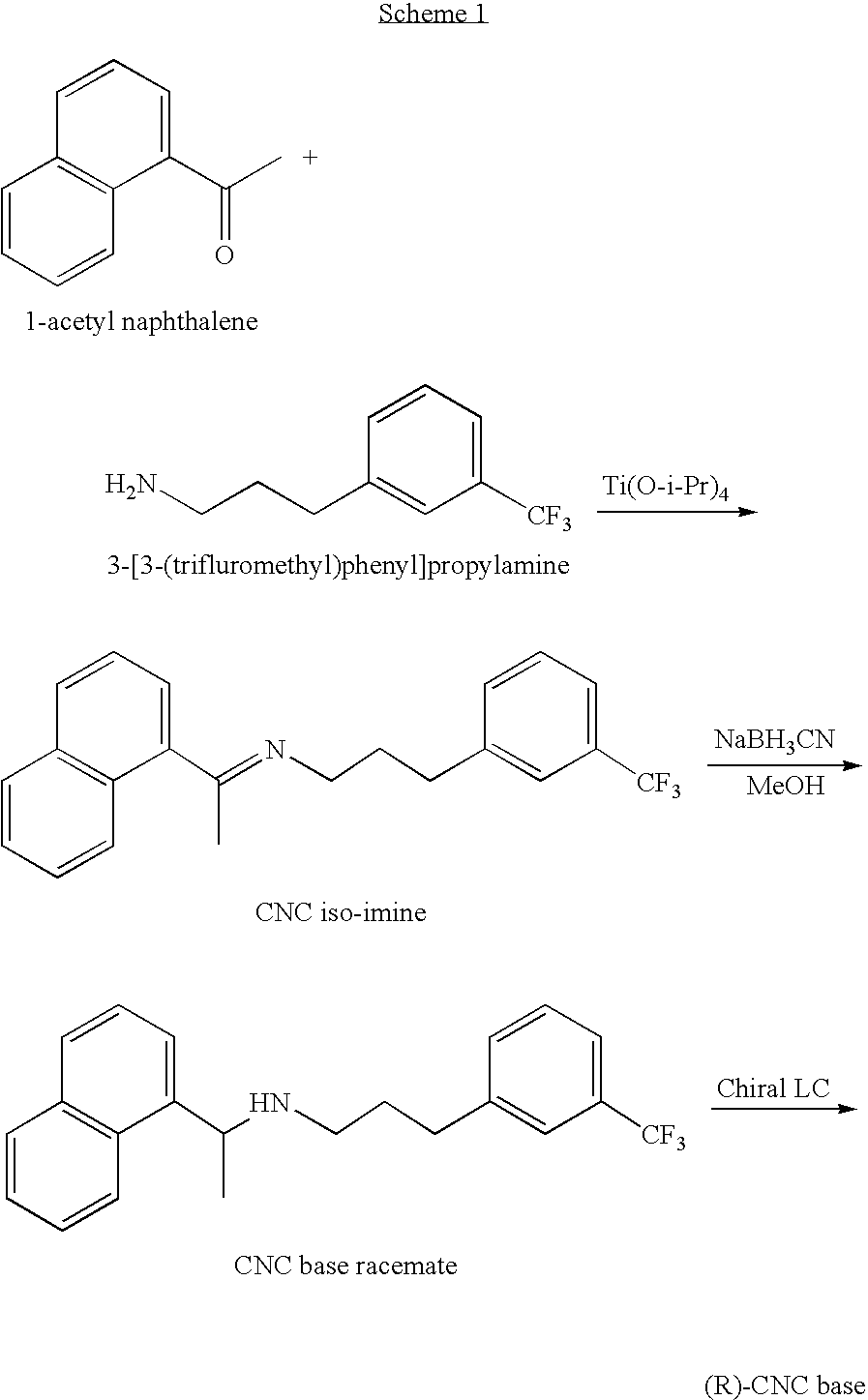
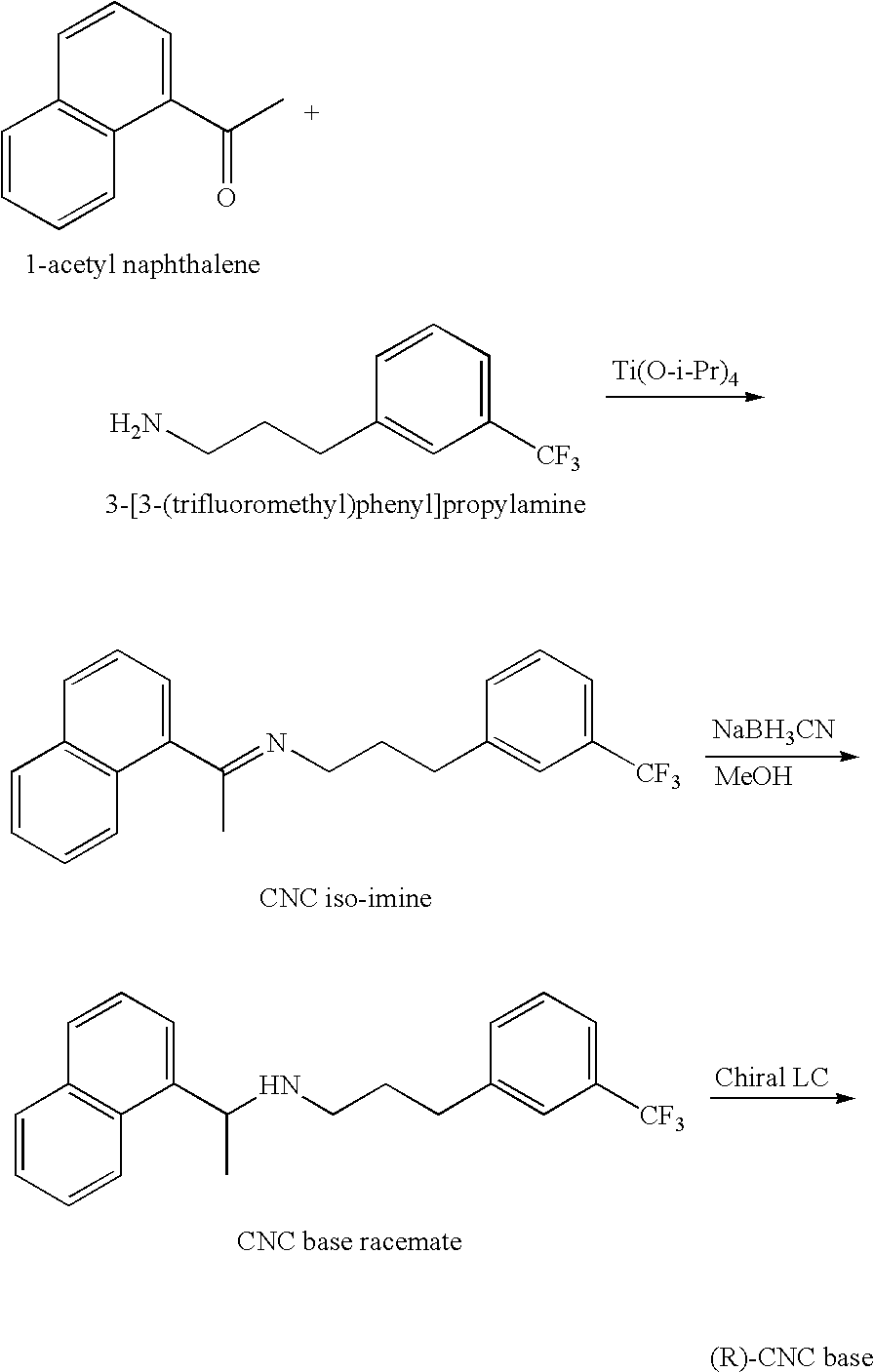
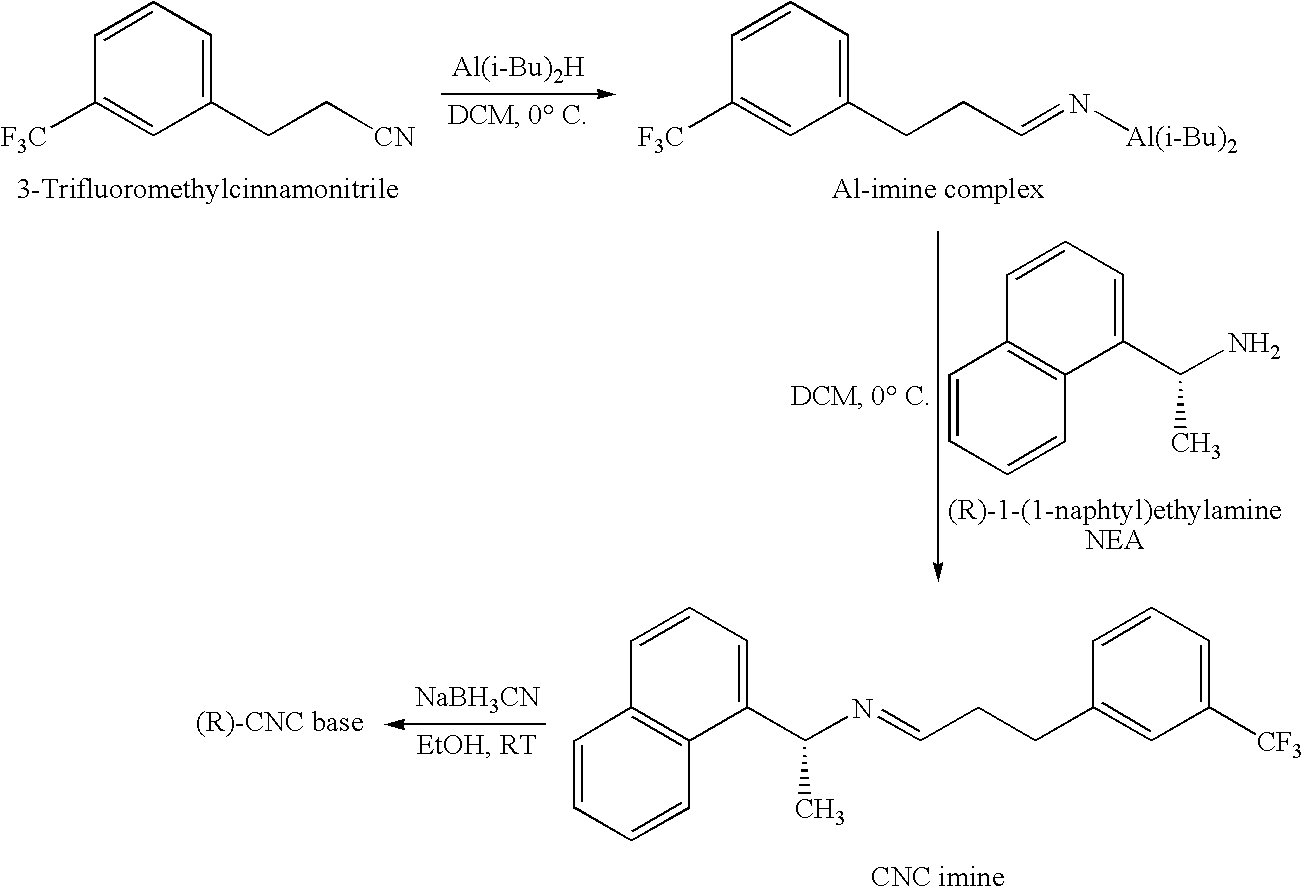
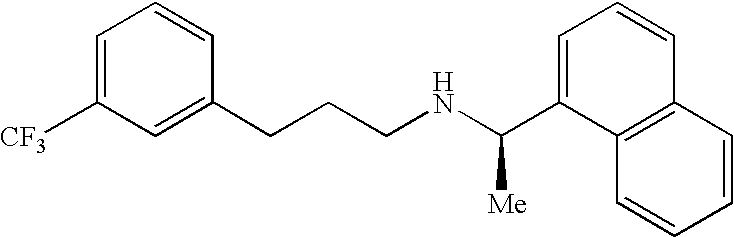
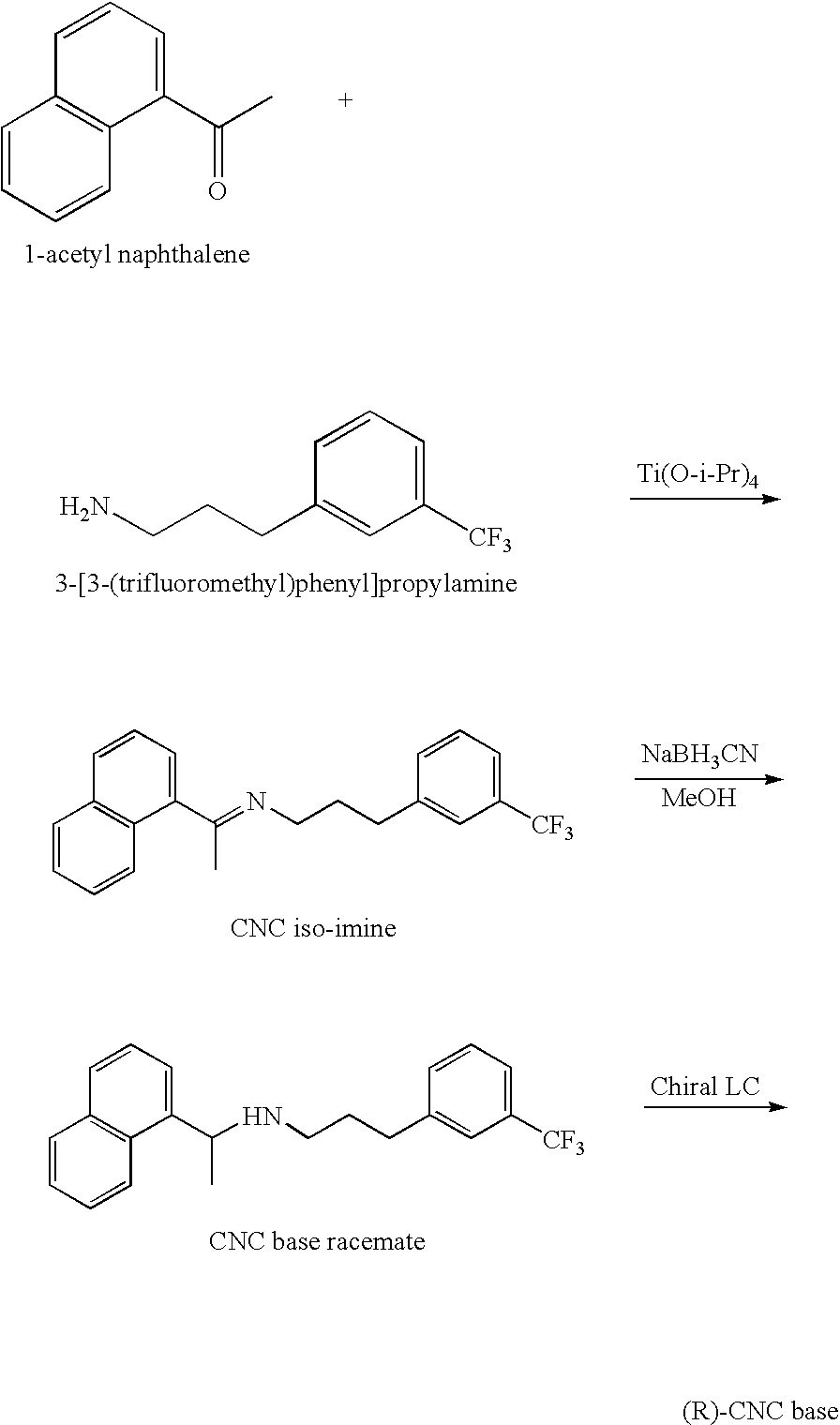
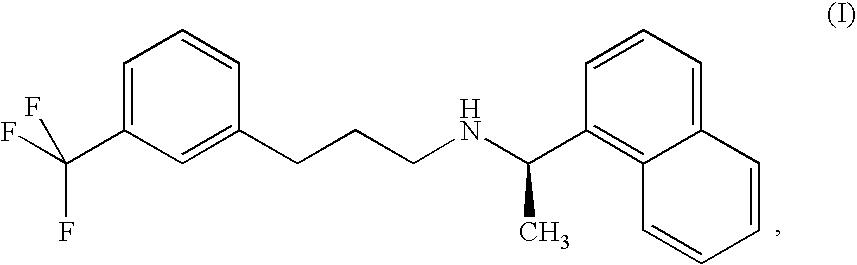
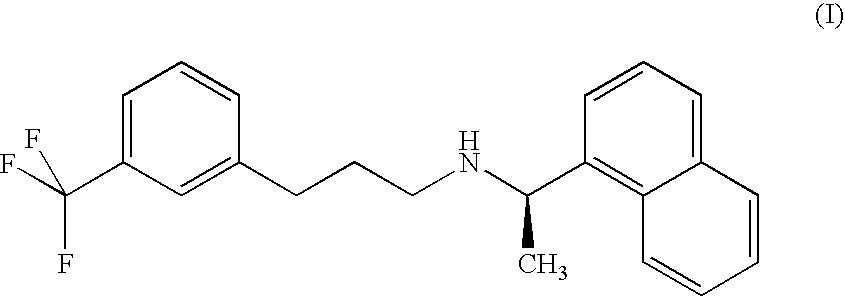
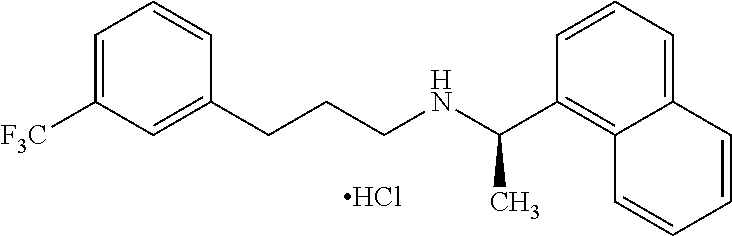
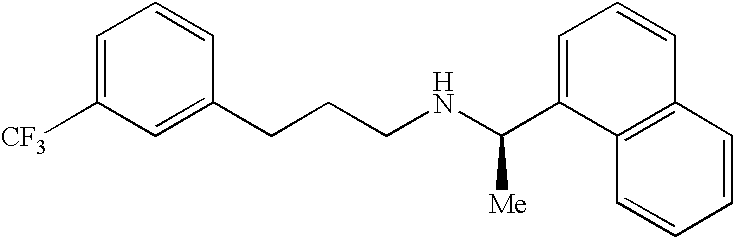
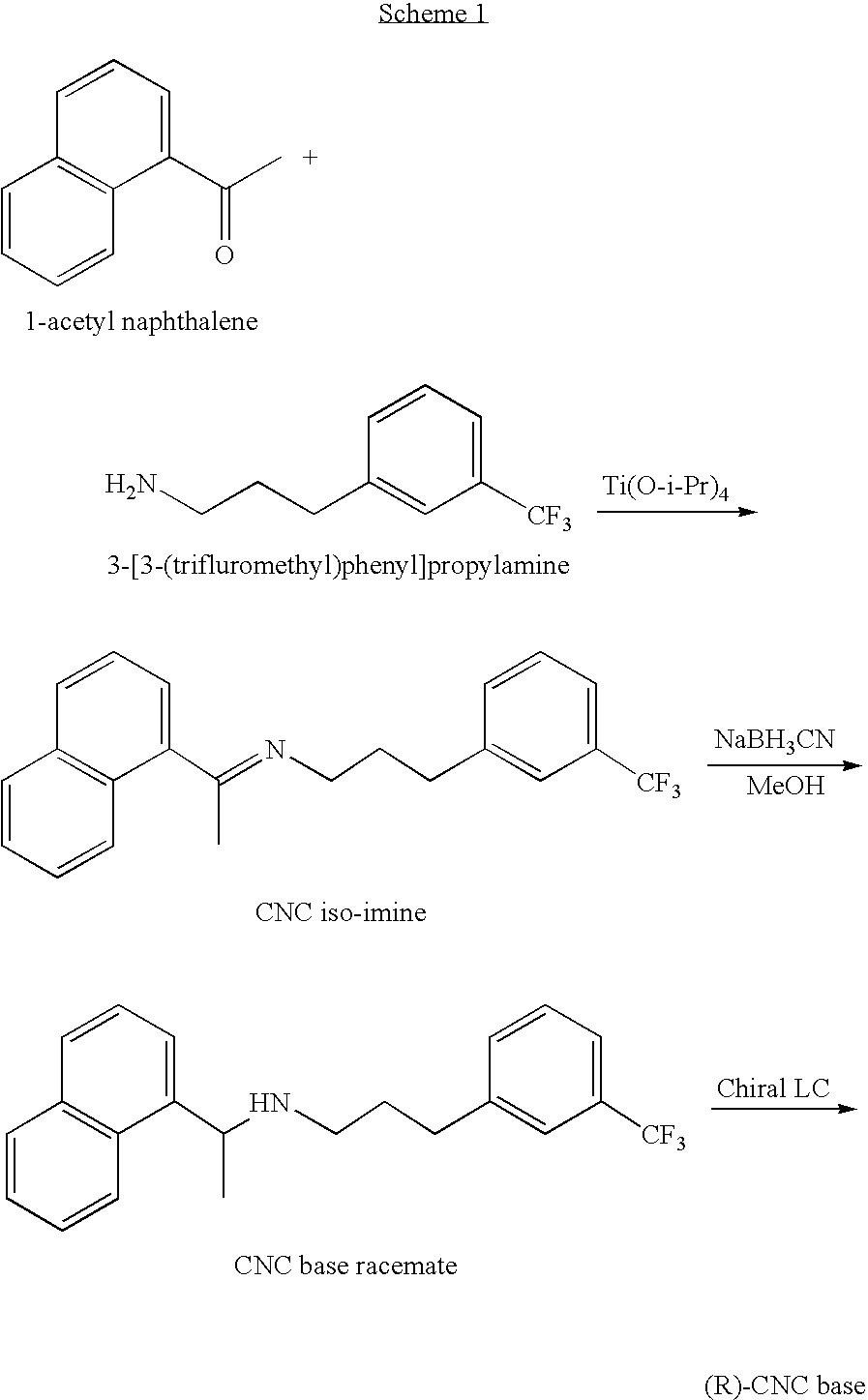
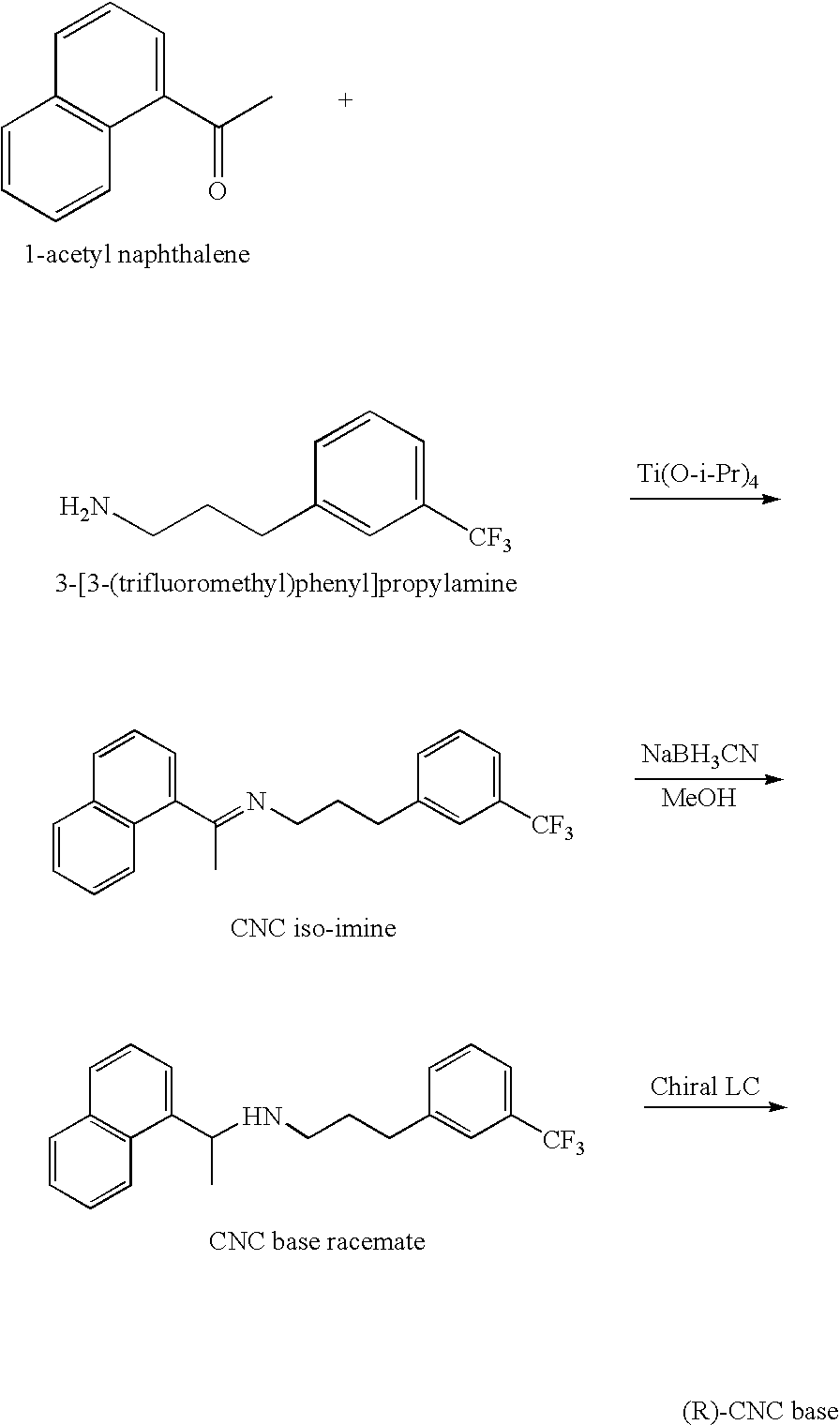
Provided is a process for preparing Cinacalcet, (R)-α-methyl-N-[3-[3-(trifluoromethyl)phenyl]propyl]-1-naphthalenemethane amine and intermediates thereof.
Owner:TEVA PHARM USA INC
Process for the preparation of cinacalcet base
Owner:TEVA PHARM USA INC
Pharmaceutical compositions comprising phosphate binder, calcium receptor-active compound and/or active vitamin d
InactiveUS20130085121A1Maintain good propertiesImprove bioavailabilityOrganic active ingredientsBiocideMineral bone diseaseCalcium Binder
The present invention is an oral solid pharmaceutical compositions for the treatment of kidney diseases and mineral bone disorder including a phosphate binder, a calcium receptor-active compound and at least one pharmaceutically acceptable excipient, the invention further including a method for preparing the pharmaceutical compositions including the steps of granulating cinacalcet and / or sevelamer and / or vitamin D by one of a wet and a dry granulation process, each with at least one pharmaceutically acceptable excipient to form cinacalcet granules and / or sevelamer granules and / or vitamin D granules, mixing at least two of the cinacalcet granules, sevelamer granules and vitamin D granules to form a granules mixture and compressing the granules mixture to tablets or encapsulating the granules mixture into capsules or pulverizing the granules mixture into a dispersion powder.
Owner:WEIFANG SYNERPHARM
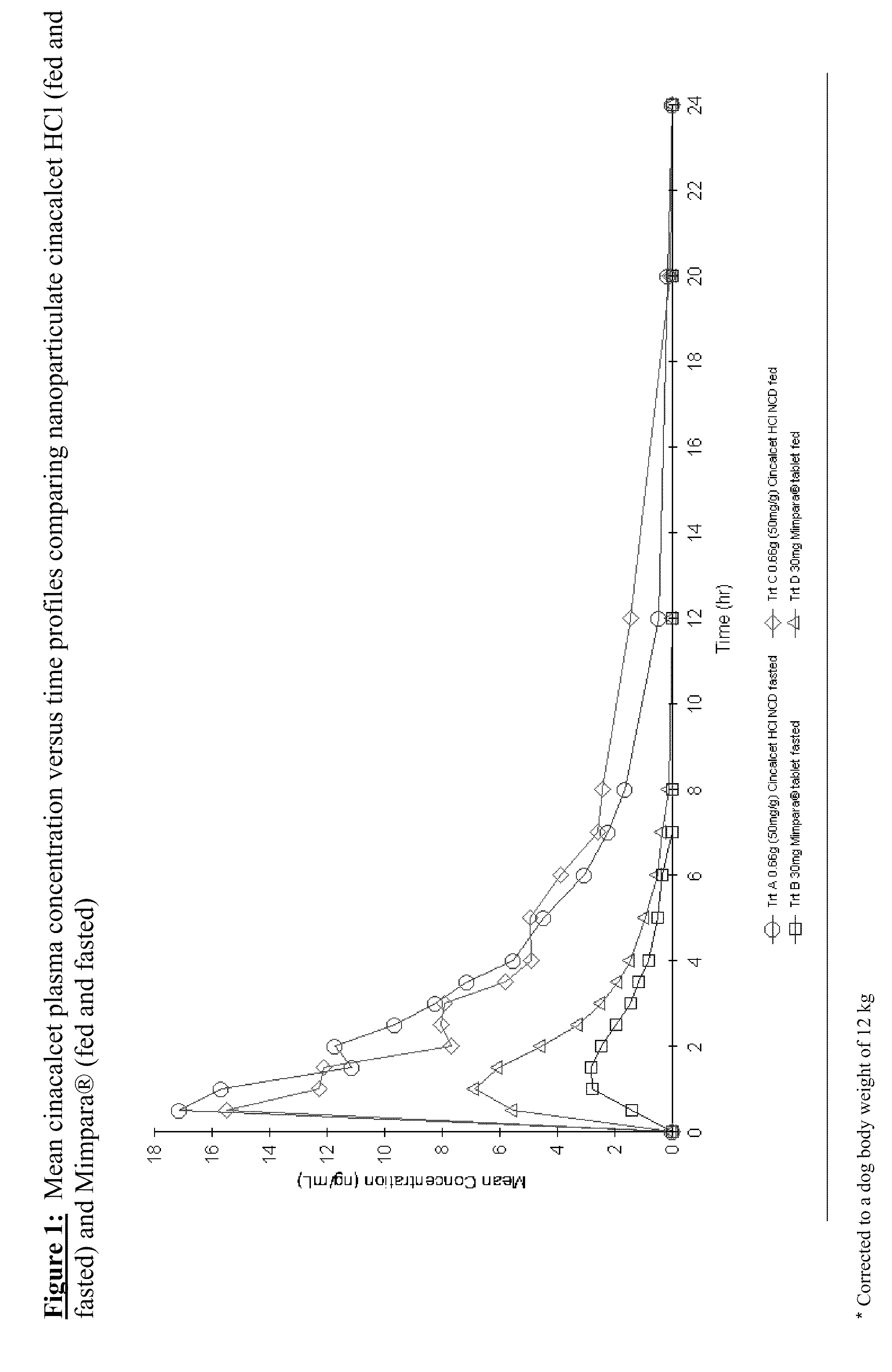
Nanoparticulate cinacalcet compositions
InactiveUS20110287065A1Improve stabilityGuaranteed effective sizeBiocidePowder deliveryMedicineNanoparticle
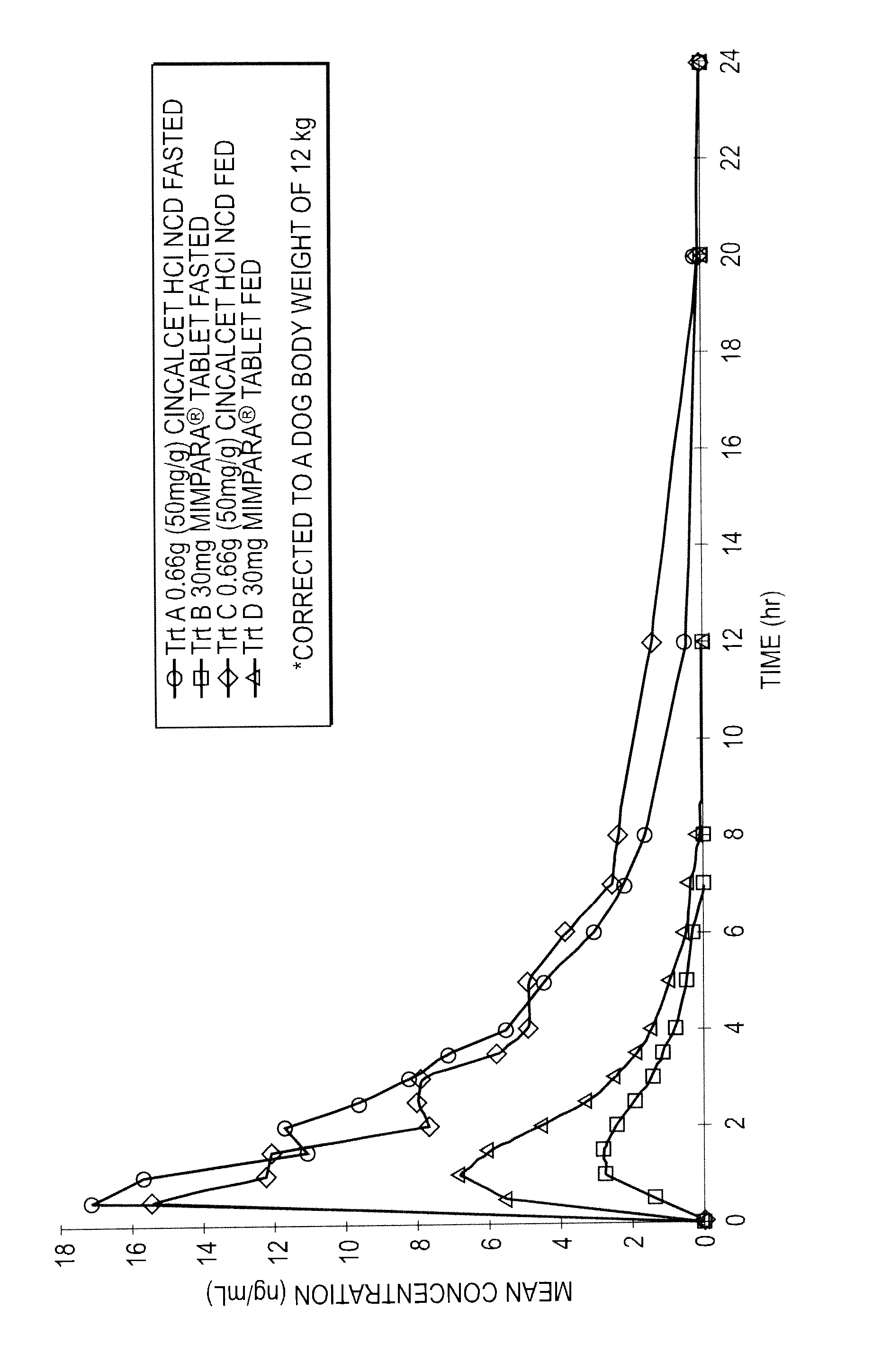
The invention is directed to compositions comprising stable nanoparticulate cinacalcet or a salt thereof. The composition can exhibit an improved dissolution rate, improved bioavailability, and reduced difference in absorption when administered orally under fed as compared to fasting conditions. The invention also encompasses methods of making and using such compositions.
Owner:ALKERMES PHARMA IRELAND LTD
Prophylactic or therapeutic composition for diabetes or obesity
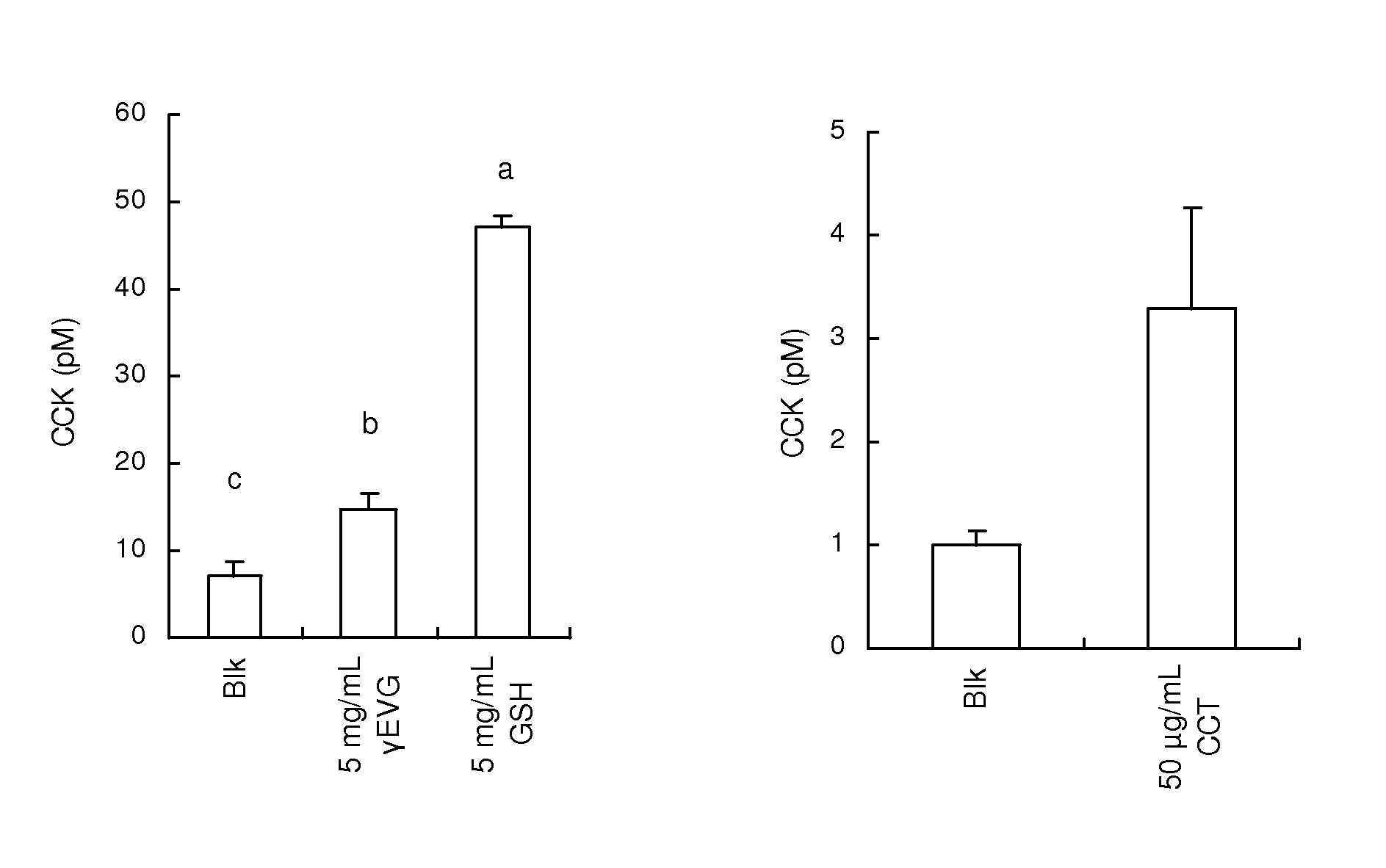
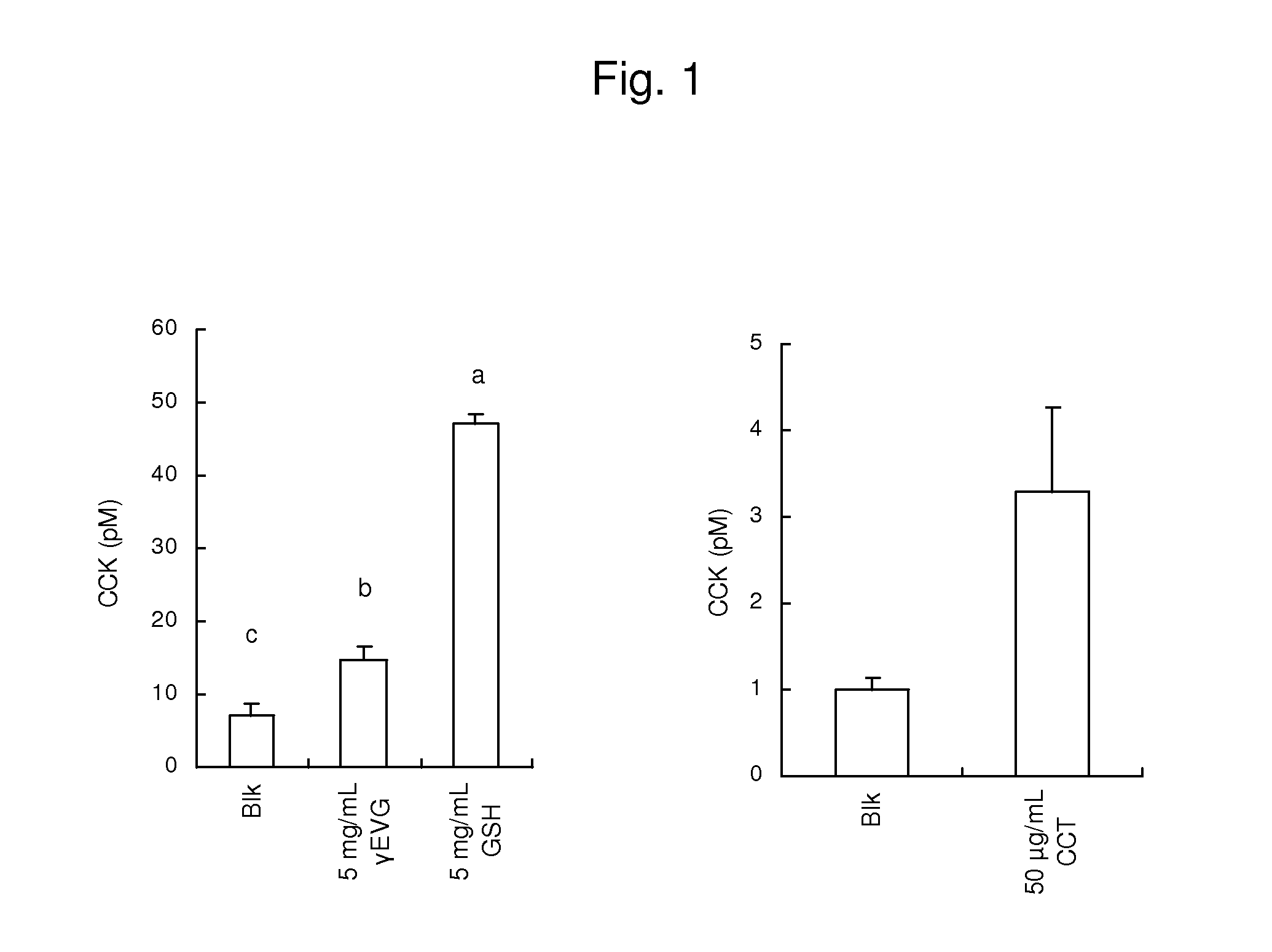
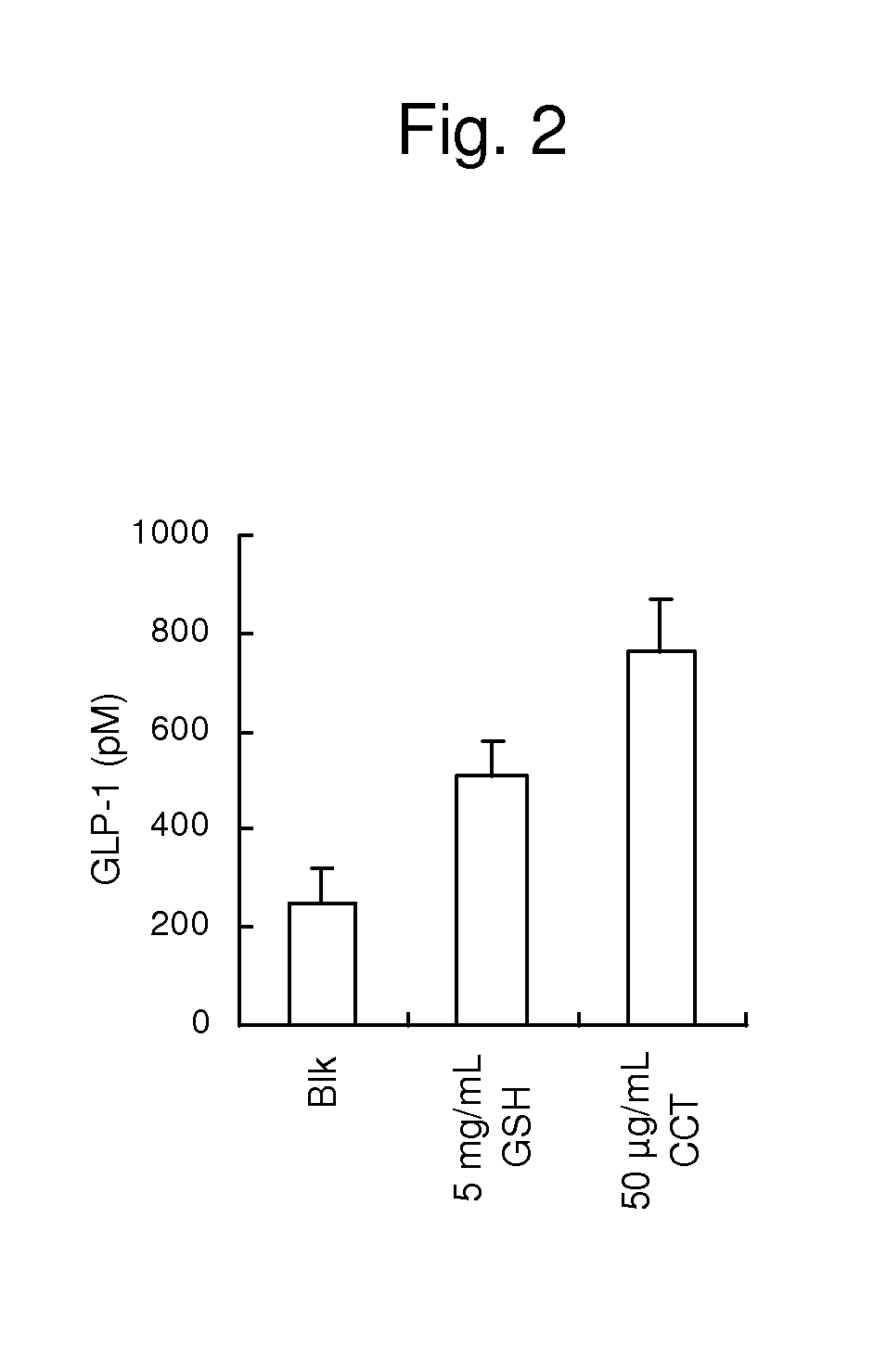
Disclosed is a safe and non-toxic prophylactic or therapeutic composition for diabetes or obesity. The prophylactic or therapeutic composition for diabetes or obesity comprises as an active ingredient, a calcium receptor activator such as γ-Glu-X-Gly, wherein X represents an amino acid or an amino acid derivative; γ-Glu-Val-Y, wherein Y represents an amino acid or an amino acid derivative; γ-Glu-Ala; γ-Glu-Gly; γ-Glu-Cys; γ-Glu-Met; γ-Glu-Thr; γ-Glu-Val; γ-Glu-Orn; Asp-Gly; Cys-Gly; Cys-Met; Glu-Cys; Gly-Cys; Leu-Asp; γ-Glu-Met(O); γ-Glu-γ-Glu-Val; γ-Glu-Val-NH2; γ-Glu-Val-ol; γ-Glu-Ser; γ-Glu-Tau; γ-Glu-Cys(S-Me)(O); γ-Glu-Leu; γ-Glu-Ile; γ-Glu-t-Leu; γ-Glu-Cys(S-Me); cinacalcet; a cinacalcet analogue compound and protamine.
Owner:AJINOMOTO CO INC +1
Method for synthesizing and refining cinacalcet hydrochlorid
ActiveCN103739500AConvenient sourceLow costAmino compound purification/separationOrganic compound preparationEngineeringEnvironmental engineering
The invention explores a method for synthesizing and refining cinacalcet hydrochlorid, especially avoids toxic and expensive reaction reagents reported in literatures; the invention has the advantages of convenient sources of raw materials and reagents, low cost, little pollution to environment and simple operation, the invention is suitable for industrial production.
Owner:SINOPHARM A THINK PHARMA
Immunostimulating agent
An immunostimulating agent, which can stimulate immunity effectively, is described. The immunostimulating agent contains an active ingredient including a calcium receptor activator such as γ-Glu-X-Gly [wherein X represents an amino acid or a derivative thereof other than Cys], γ-Glu-Val-Y [wherein Y represents an amino acid or a derivative thereof], γ-Glu-Ala, γ-Glu-Gly, γ-Glu-Met, γ-Glu-Thr, γ-Glu-Val, γ-Glu-Orn, Asp-Gly, Cys-Gly, Cys-Met, Glu-Cys, Gly-Cys, Leu-Asp, γ-Glu-Met(O), γ-Glu-γ-Glu-Val, γ-Glu-Val-NH2, γ-Glu-Val-ol, γ-Glu-Ser, γ-Glu-Tau, γ-Glu-Cys(S-Me)(O), γ-Glu-Leu, γ-Glu-Ile, γ-Glu-t-Leu, γ-Glu-Cys(S-Me), a cation having a valency of 2 or more, protamine, polylysine, spermine, spermidine, putrescine, cinacalcet, a cinacalcet analogue compound, and a salt of any one of the aforementioned components.
Owner:AJINOMOTO CO INC
Oral solid rapid release preparation of cinacalcet hydrochloride
InactiveCN102885792AIncrease dissolution rateHigh dissolution rateOrganic active ingredientsPill deliveryDissolutionCinacalcet Hydrochloride
The invention discloses an oral solid rapid release preparation of cinacalcet hydrochloride. The oral solid rapid release preparation comprises cinacalcet hydrochloride and medicinal auxiliary materials, wherein the average particle diameter of cinacalcet hydrochloride is 5-15 mum; the cinacalcet hydrochloride is needlelike crystalized fine particles; the medicinal auxiliary materials include a filling agent, a bonding agent, a disintegrating agent and a lubricating agent; and the preparation is in the form of a tablet. The oral solid rapid release preparation has the beneficial effects that: the average particle diameter of cinacalcet hydrochloride is defined, so that the dissolution rate and dissolution amount of cinacalcet are increased, a preparation process is simple and safe, the quality is controllable, and cost is low; and the oral solid rapid release preparation is suitable for industrial production.
Owner:CHINA RESOURCES SAIKE PHARMA
Process for preparing Cinacalcet hydrochloride tablets or capsules
InactiveCN102198108ASolve the preparation processOrganic active ingredientsPharmaceutical non-active ingredientsCinacalcet HydrochloridePharmacology
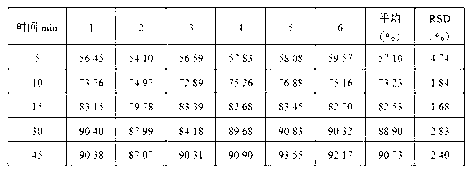
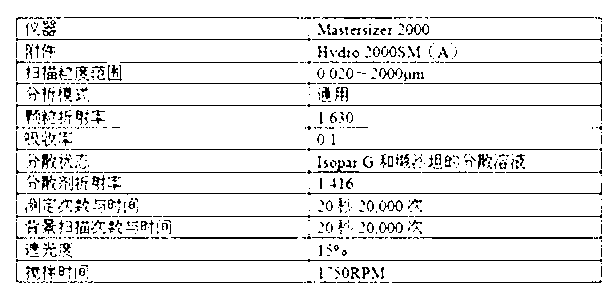
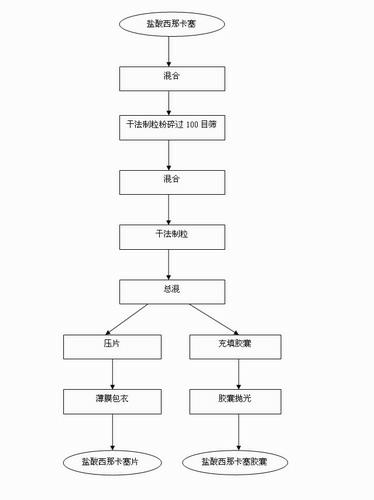
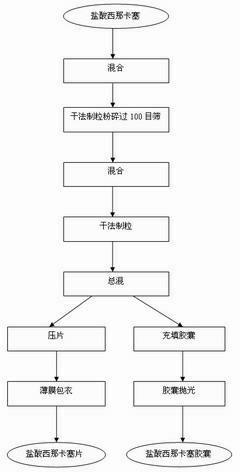
The invention discloses a process for preparing Cinacalcet hydrochloride tablets or capsules and belongs to the technical field of medicinal preparations. The preparation process comprises performing dry-process granulation according to one formula; and tabletting or filling capsules by using three dosages according to a ratio. The in-vitro release of the Cinacalcet hydrochloride is characterized in that: 40 to 80 percent of Cinacalcet hydrochloride is released within 5 to 10 minutes; 80 to 90 percent of the Cinacalcet hydrochloride is released within 15 to 20 minutes; and 90 to 100 percent of Cinacalcet hydrochloride is released within 30 to 45 minutes. In the process, the procedures are simple and safety, the quality is controllable, the cost is low, and the production period is short. The process is suitable for large-scale industrial production.
Owner:四川晖瑞医药科技有限公司
Process for the preparation of cinacalcet
InactiveUS20080319229A1Organic compound preparationAmino compound preparationCinacalcetMedicinal chemistry
Owner:DIPHARMA FRANCIS
Environment-friendly synthesis method for cinacalcet
InactiveCN101941911AReduce pollutionSimple and fast operationAmino preparation by functional substitutionPtru catalystMeth-
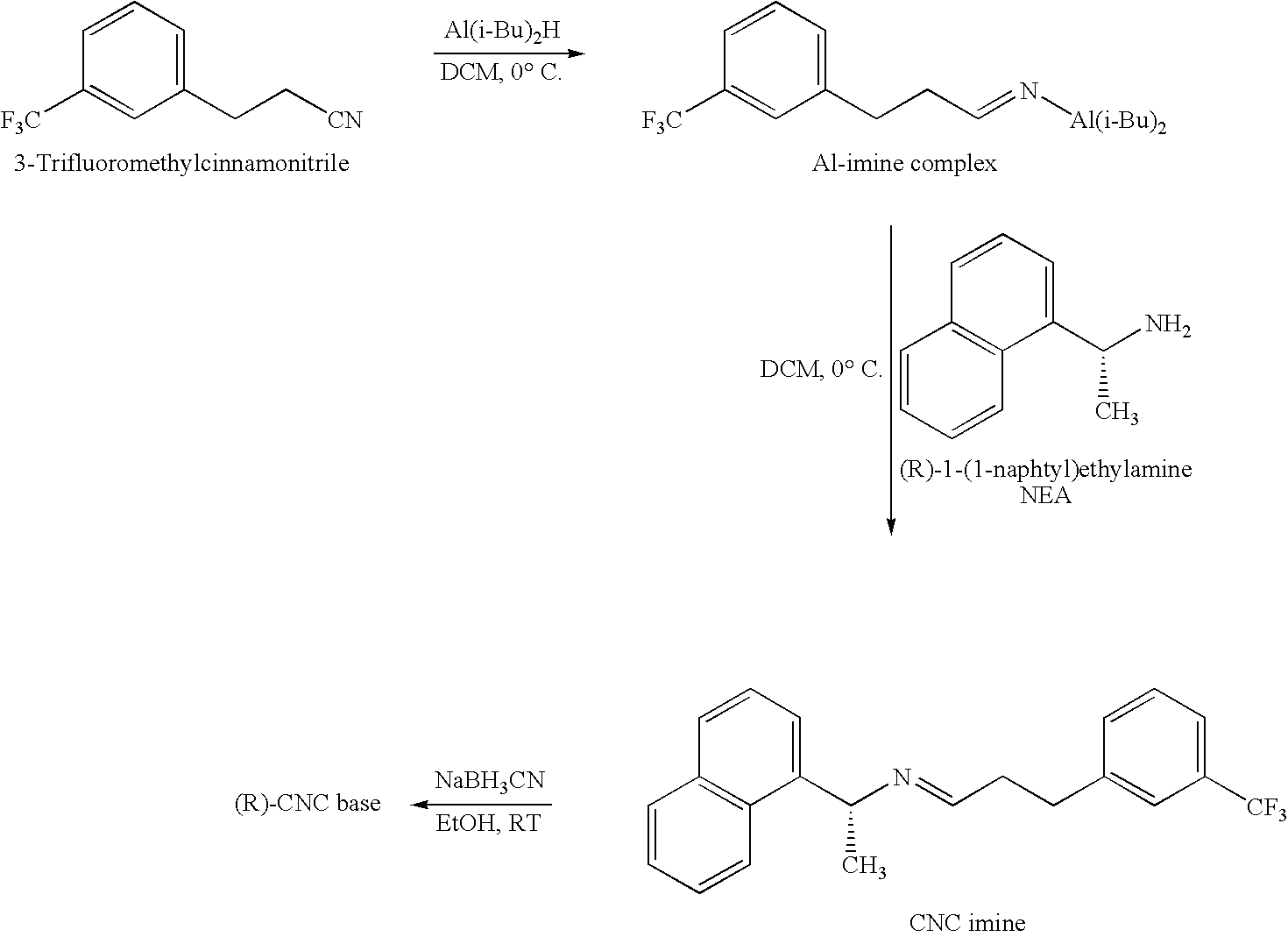
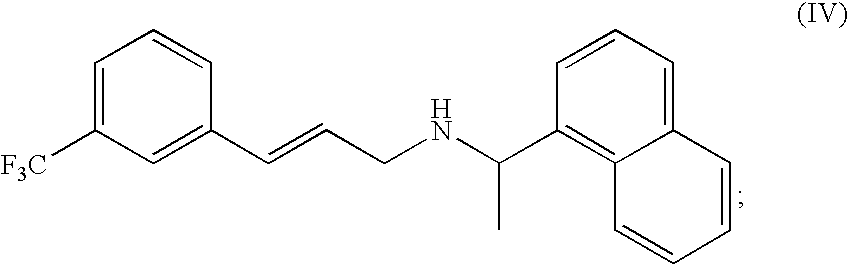
The invention discloses an environment-friendly synthesis method for cinacalcet. The cinacalcet is prepared by reacting R(+)-1-naphthyl ethamine and [3-(3'-trifluoromethyl)phenyl]-1-halopropane or [3-(3'-trifluoromethyl)phenyl]-1-propylsulfonate serving as raw materials with water serving as medium under the action of a phase-transfer catalyst. The process has the advantages of reducing the cost, along with high yield, no any organic solvent during the reaction, and low environmental pollution, and thus is a synthesis method suitable for industrialization.
Owner:SHANGHAI INSTITUTE OF TECHNOLOGY
Cinacalcet hydrochloride preparation method
InactiveCN104478736ALow impurity contentNo pollution in the processOrganic compound preparationAmino compound preparationPalladium on carbonCinacalcet Hydrochloride
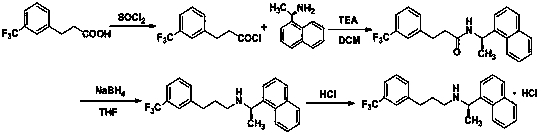
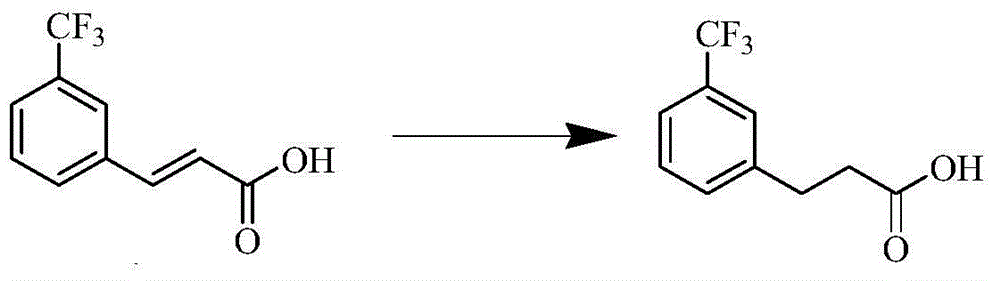
The invention provides a cinacalcet hydrochloride preparation method which comprises the following steps: (A) by taking trifluoromethylcinnamic acid as a raw material and palladium on carbon as a catalyst, performing hydrogenation reduction to obtain m-trifluoromethylbenzoic acid; (B) performing condensation reaction on the m-trifluoromethylbenzoic acid and R-(1) naphthylethylamine, thus obtaining (R)-N-(1-(naphthalen-1-yl)ethyl)-3-(trifluoromethyl)phenyl)propanamide; (C) performing catalytic reduction on the (R)-N-(1-(naphthalen-1-yl)ethyl)-3-(trifluoromethyl)phenyl)propanamide to obtain cinacalcet; and (D) reacting the cinacalcet and hydrochloric acid to obtain cinacalcet hydrochloride. The method has the advantages of thorough reaction, high yield, no pollution in the reaction process and the like.
Owner:CHENGDU QITAI PHARMA TECH
Cinacalcet hydrochloride preparation method
InactiveCN103467304AOvercoming technical biases usedOvercoming technical biasAmino compound purification/separationPreparation by reductive alkylationSolventPollution
The present invention discloses a cinacalcet hydrochloride preparation method, which comprises: 1, adopting 3-(trifluoromethyl) phenyl propionaldehyde and R-1-(1-naphthyl)ethylamine as raw materials, and carrying out a condensation reduction reaction in a mixing reaction solvent in the presence of sodium triacetoxyborohydride, 2, extracting the reaction product with water, a salt or an alkali aqueous solution to obtain a cinacalcet base organic phase solution, and 3, carrying out acidification salification on the obtained cinacalcet base organic phase solution with hydrochloric acid to obtain the cinacalcet hydrochloride, wherein one of components in the mixing reaction solvent is water. According to the present invention, the water-containing mixing solvent is adopted as the reaction medium, such that technical defects of solvent use during the sodium triacetoxyborohydride use process are overcome, the harsh condition that the reaction solvent requires a water-free treatment is avoided while reaction selectivity is increased, types of produced impurities and impurity content are substantially reduced, cost is reduced, and environment pollution is reduced.
Owner:NANJING LIFENERGY R & D
Nanoparticulate cinacalcet compositions
InactiveUS9012511B2Improve stabilityGuaranteed effective sizePowder deliveryBiocideOral medicationCinacalcet
Described are compositions of stable nanoparticulate cinacalcet or a salt thereof, and methods of making and using them. The compositions exhibit an improved dissolution rate, improved bioavailability, and reduced difference in absorption when administered orally under fed as compared to fasting conditions.
Owner:ALKERMES PHARMA IRELAND LTD
Process for preparing Cinacalcet hydrochloride
InactiveUS20070043243A1Organic compound preparationOrganic chemistry methodsCinacalcetCinacalcet Hydrochloride
Owner:TEVA PHARM USA INC
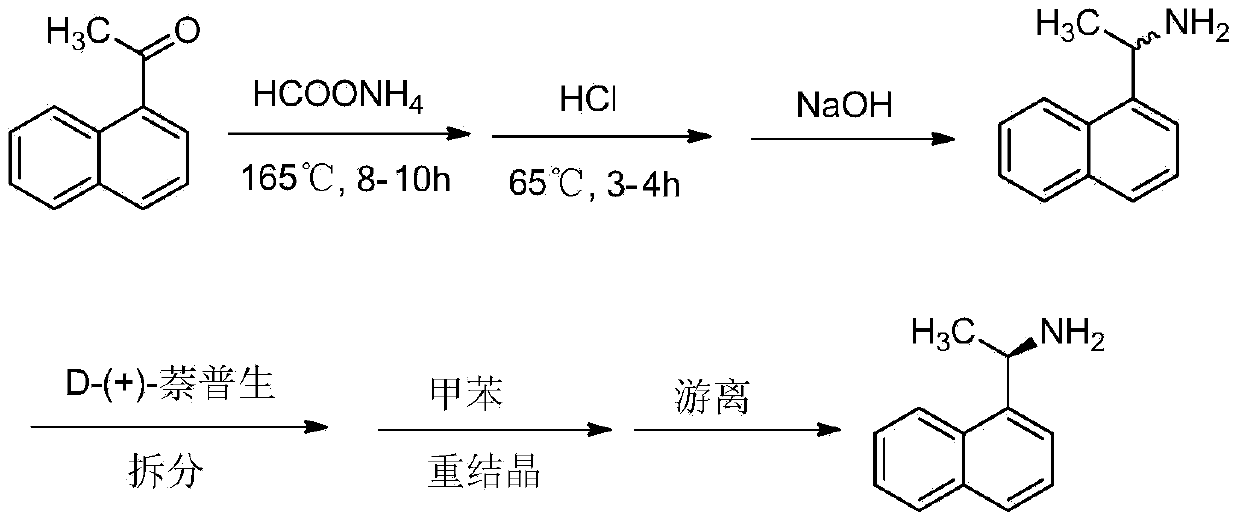
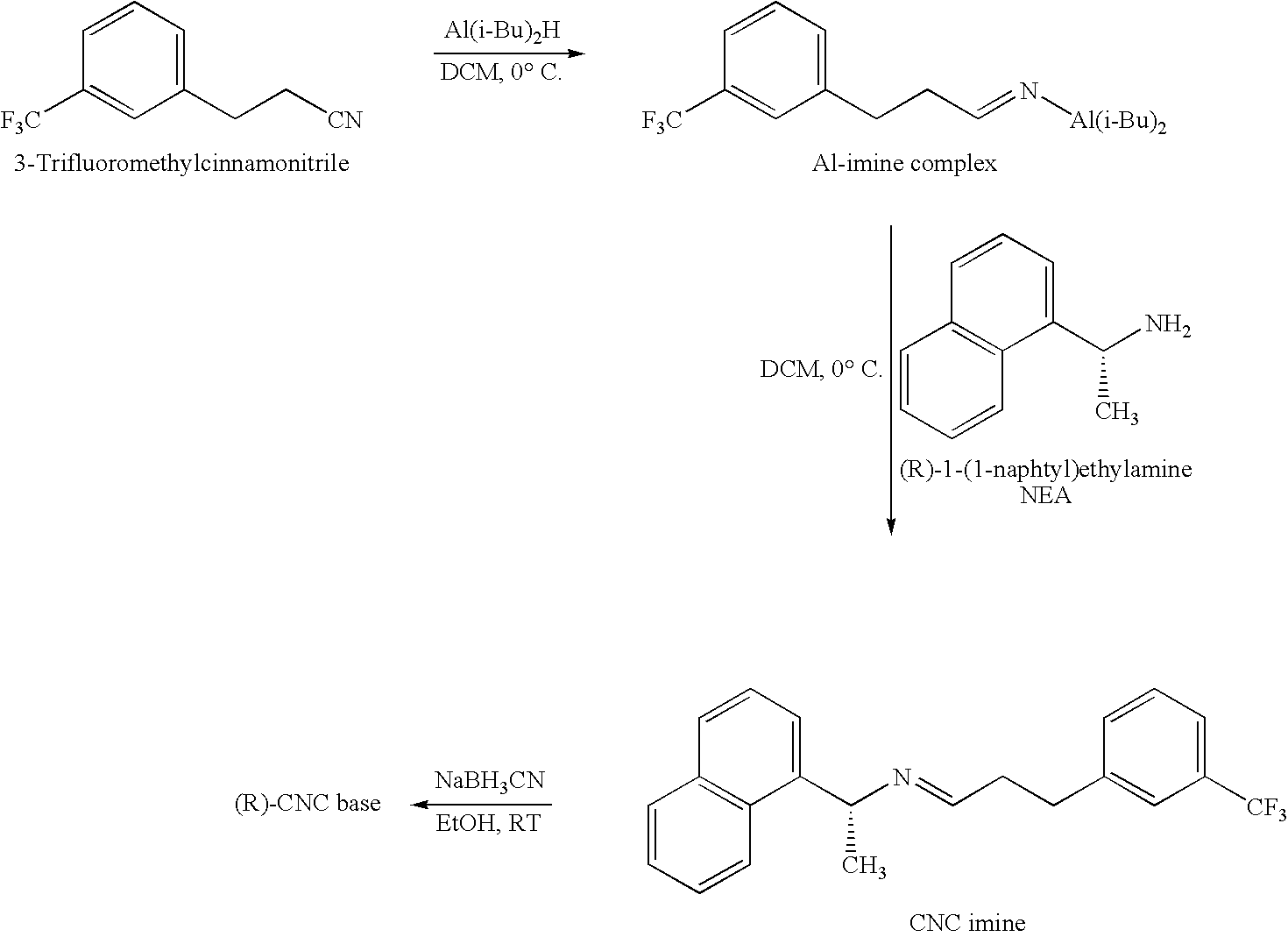
Method for preparing cinacalcet intermediate R-(+)-1-(1-naphthyl)ethamine
ActiveCN103420845ALow costMeet the requirements of green chemistryAmino compound purification/separationDrugs synthesisCinacalcet
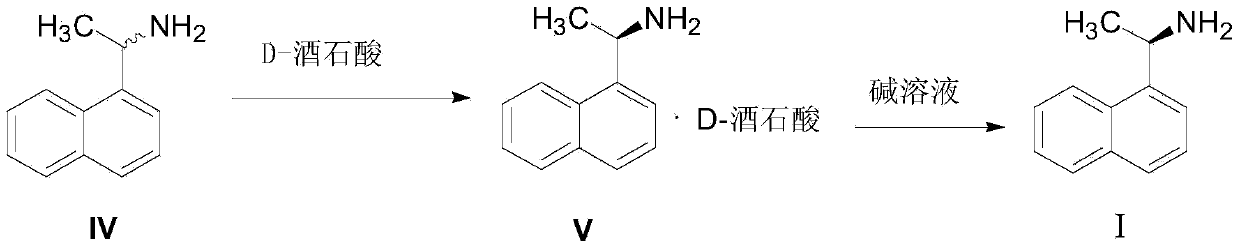
The invention relates to the field of drug synthesis, in particular to a method for preparing a cinacalcet intermediate R-(+)-1-(1-naphthyl)ethamine. The method is characterized in that D-tartaric acid which is low in price and easy to obtain is selected to replace D-(+)-naproxen to serve as a resolving agent, e.e. of an obtained product is up to 99.9%, and the method has the advantages of low cost, environmental friendliness and the like.
Owner:CHINA PHARM UNIV +1
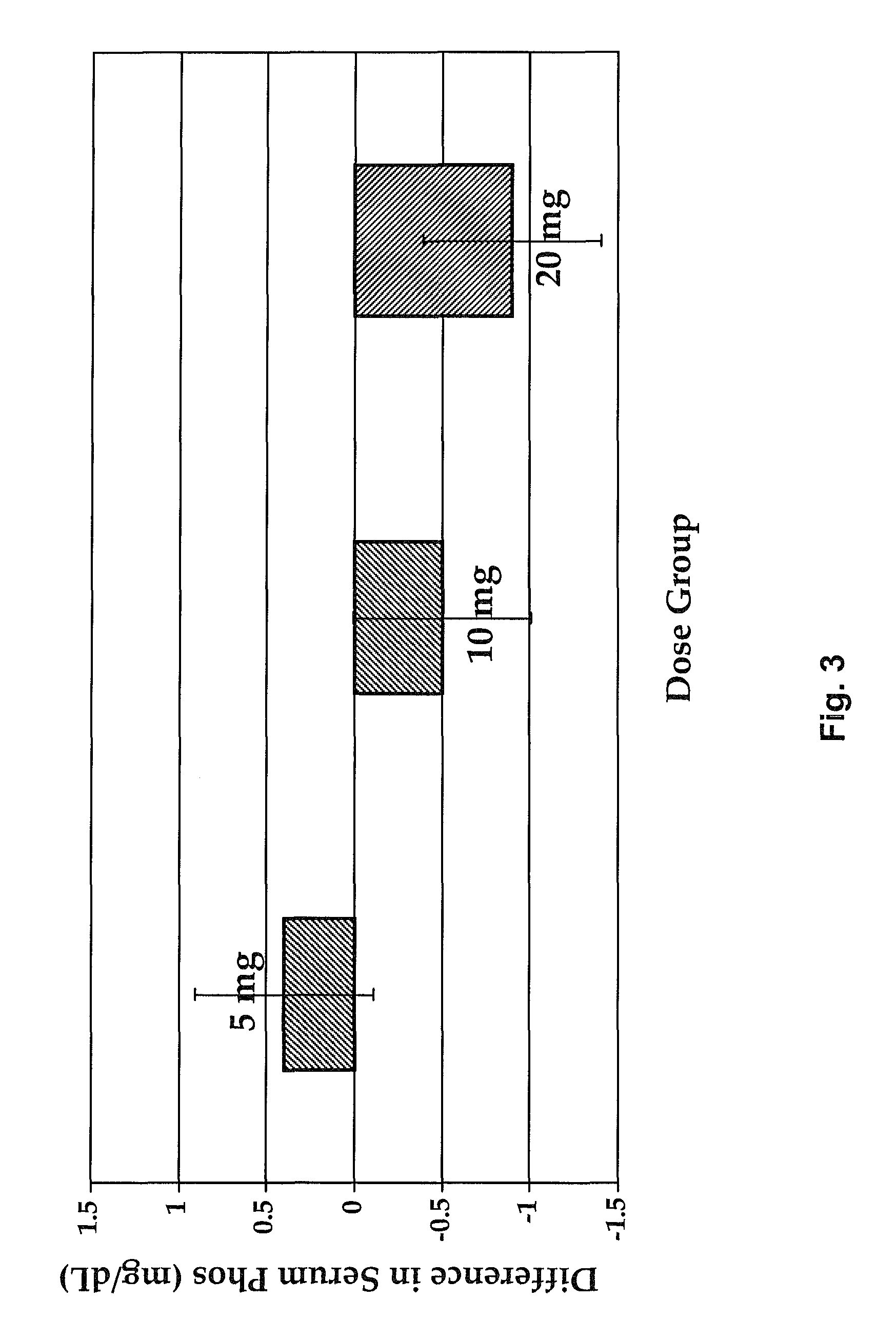
Therapeutic agents for regulating serum phosphorus
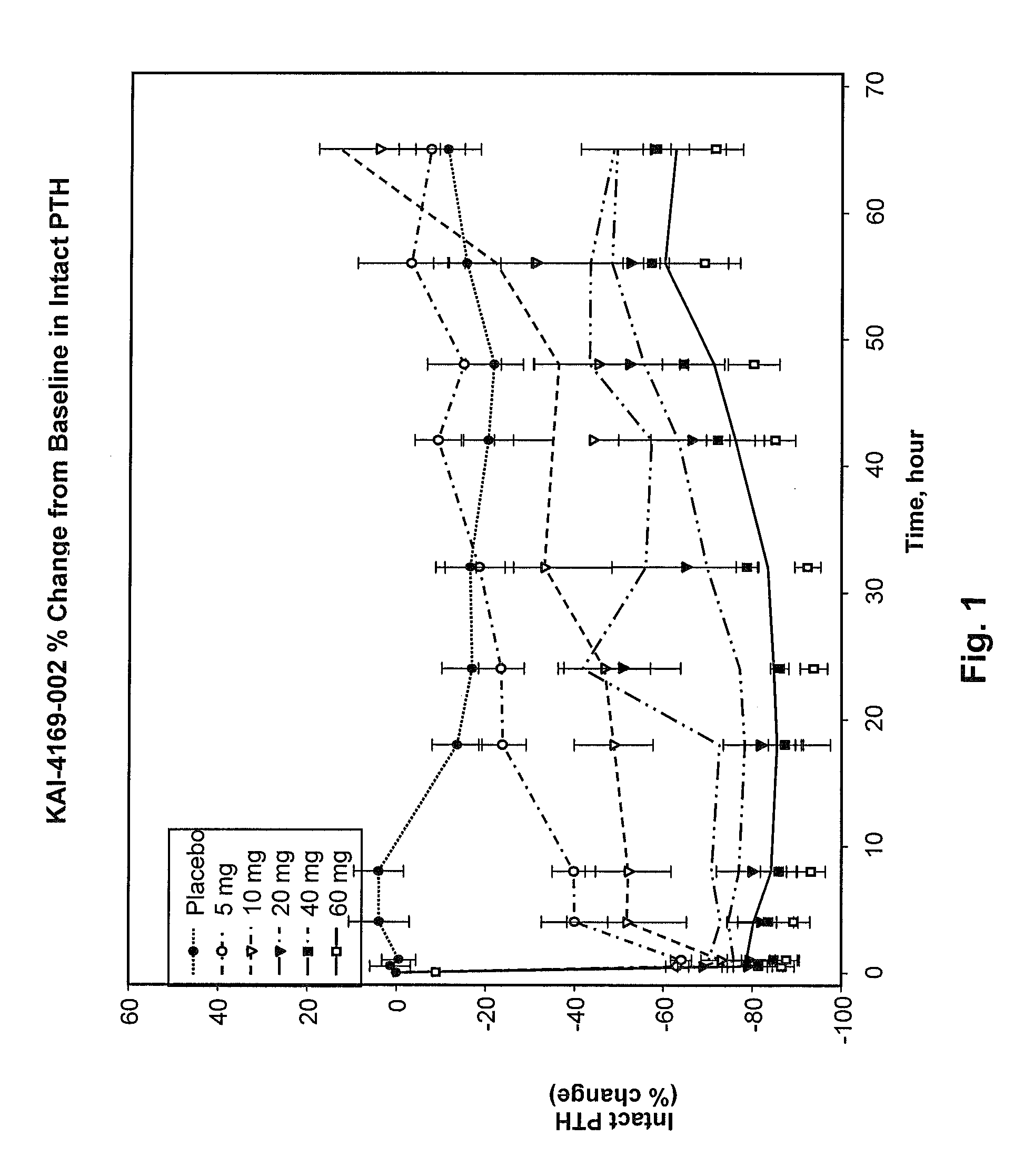
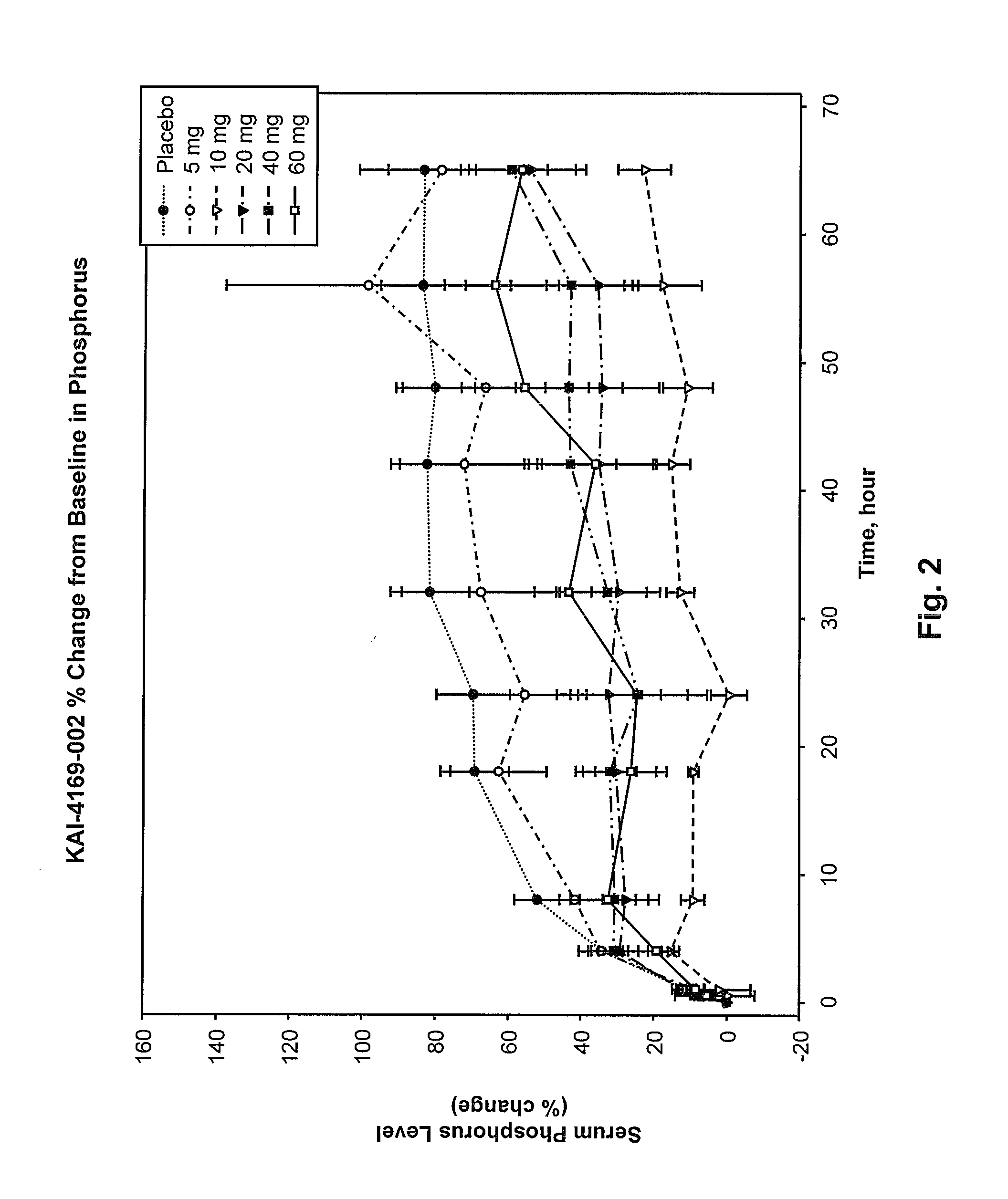
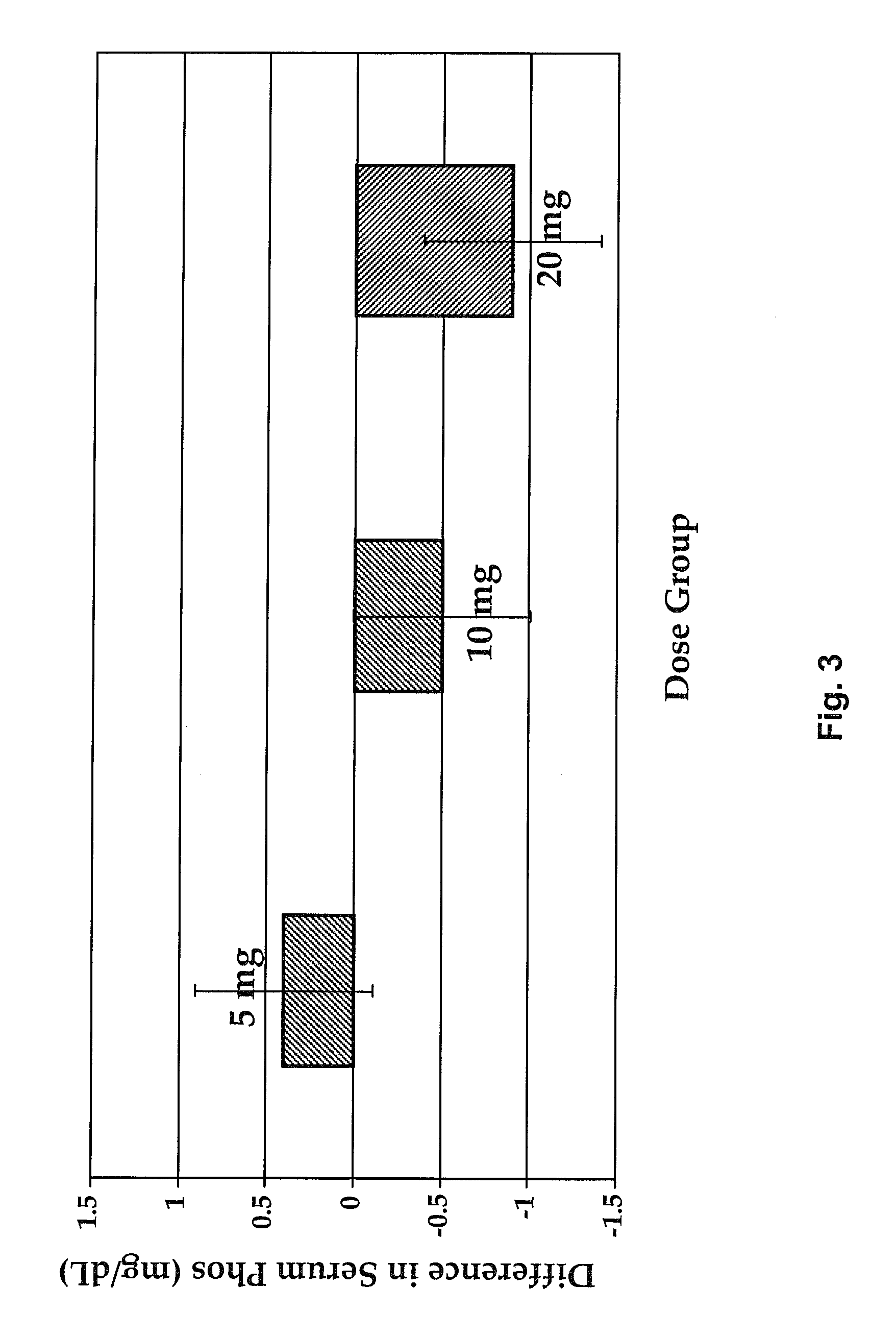
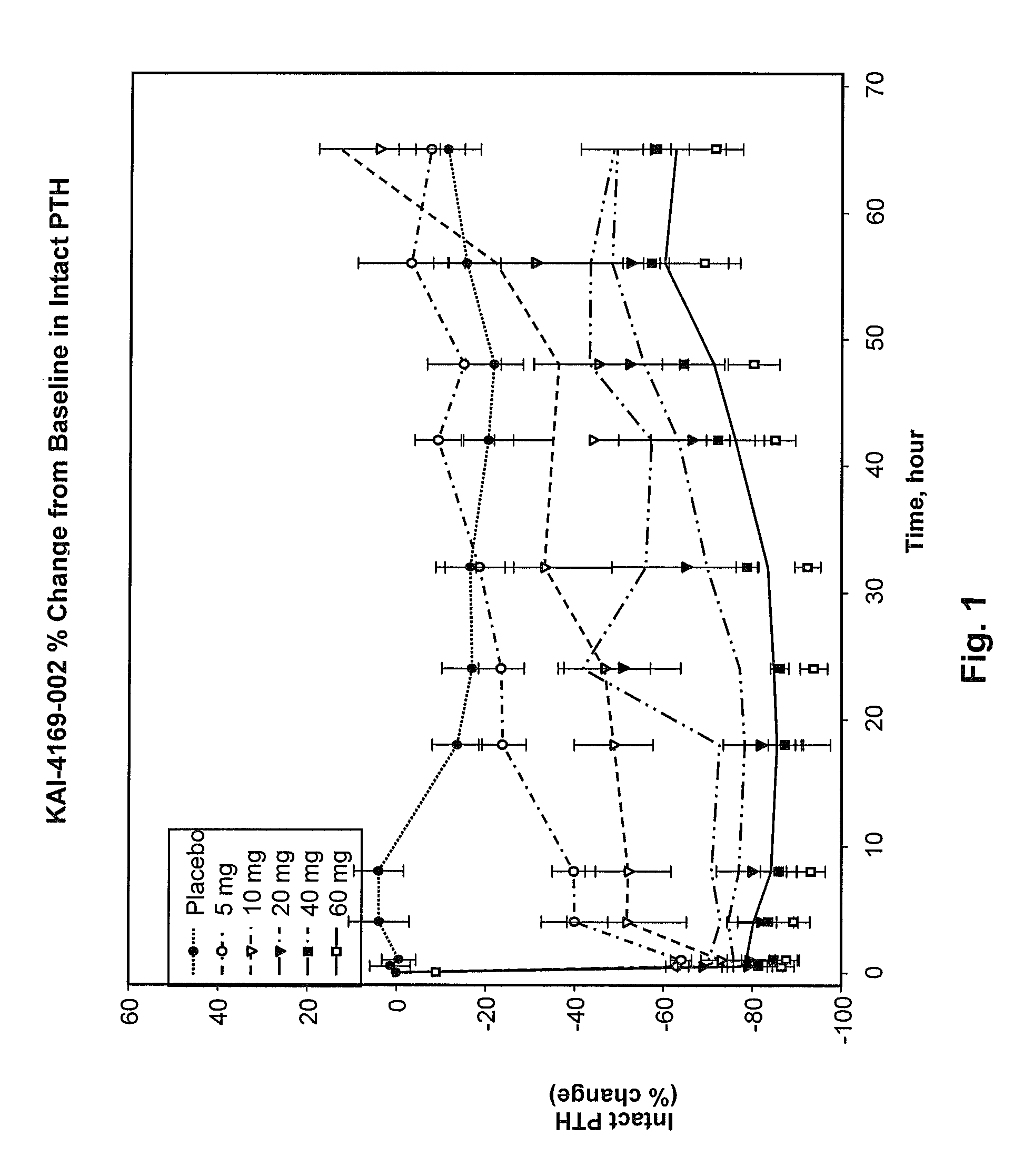
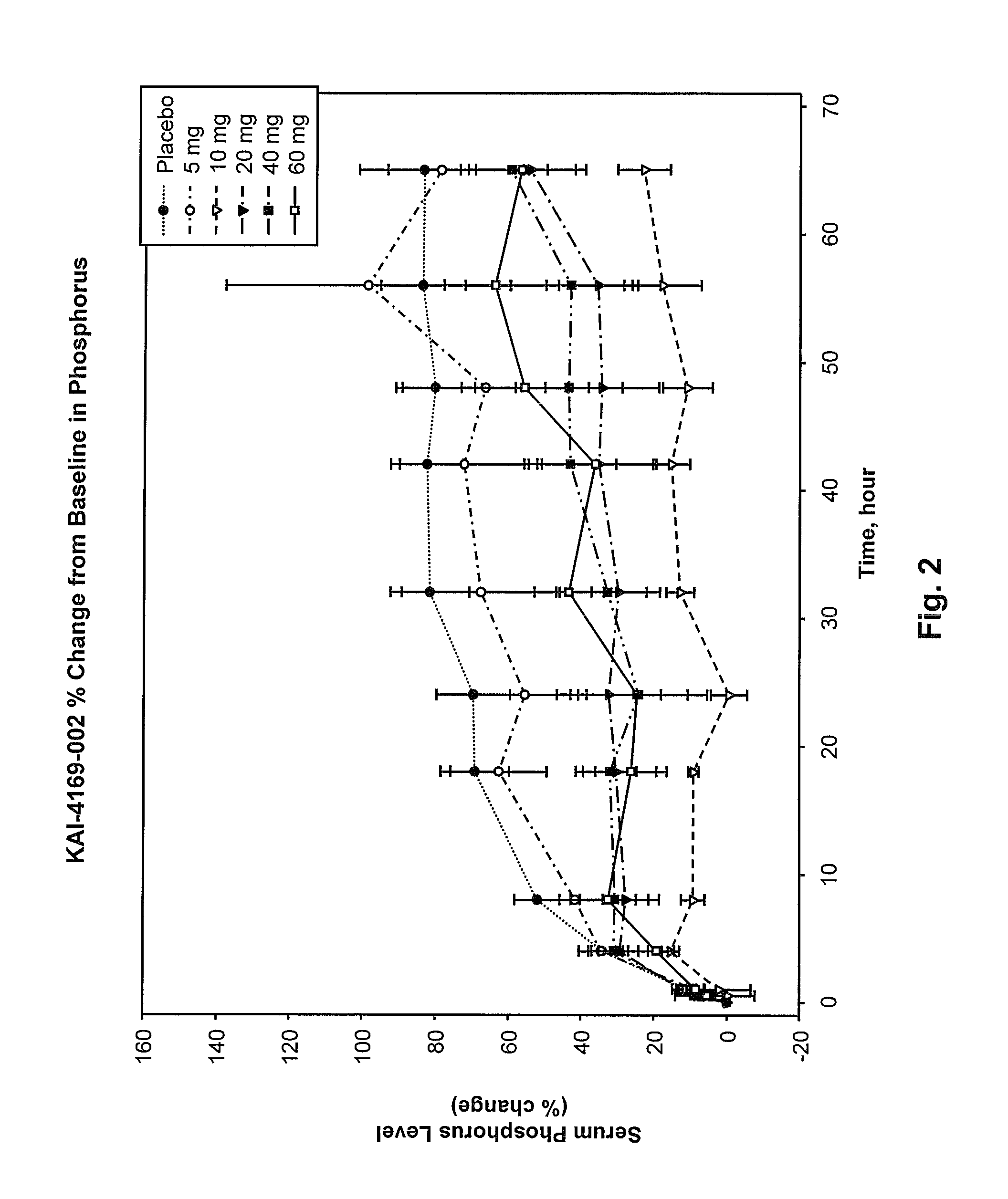
Methods for modulating serum phosphorus levels are described, wherein calcimimetic agents are administered to a subject in need thereof. In one embodiment, the compound is cinacalcet, and in other embodiments the compound is comprised of a contiguous sequence of subunits, X1—X2—X3—X4—X5—X6—X7, wherein the X1 subunit comprises a thiol-containing moiety and the distribution of charge on the X2-X7 subunits. The compound, when administered at selected times to a patient undergoing dialysis, lowers serum phosphorus levels, relative to pre-dosing levels, and achieves a sustained reduced level for a period of time after administration.
Owner:KAI PHARMA
Process for the preparation of cinacalcet base
InactiveUS20070260091A1Carbamic acid derivatives preparationOrganic compound preparationCinacalcetOrganic chemistry
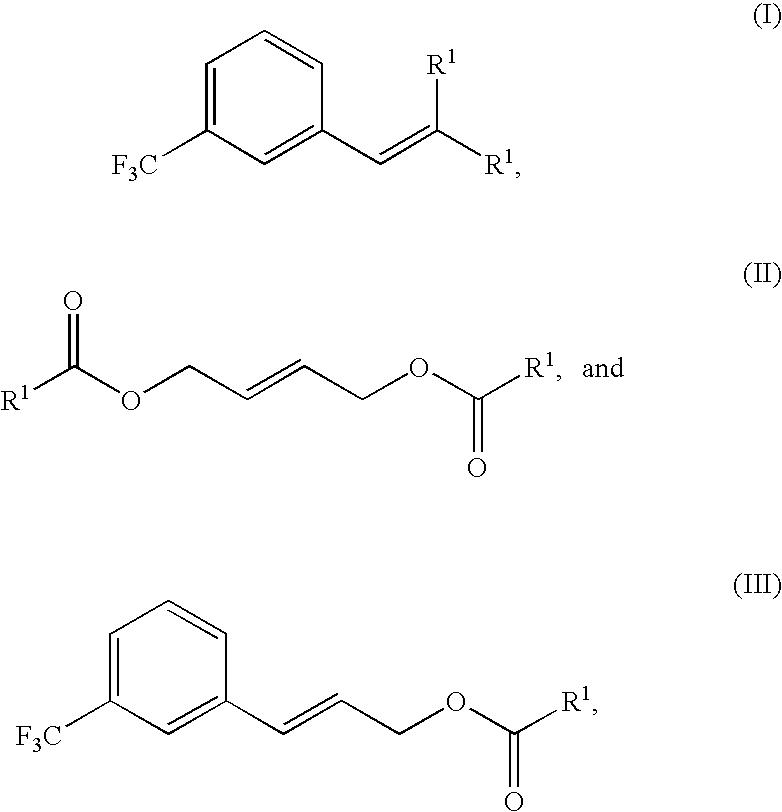
Provided is a process for preparing cinacalcet, (R)-α-methyl-N-[3-[3-(trifluoromethyl)phenyl]propyl]-1-naphthalenemethane amine.
Owner:TEVA PHARM USA INC
Preparation method of cinacalcet intermediate
ActiveCN103664577AHigh yieldEasy to purifyOrganic compound preparationPreparation from carboxylic acid esters/lactonesCombinatorial chemistryCinacalcet
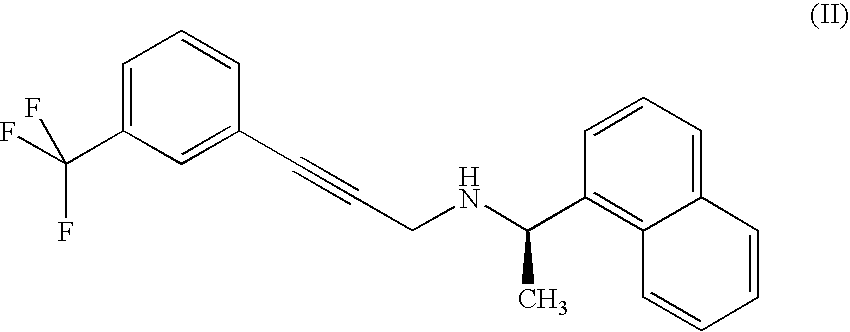
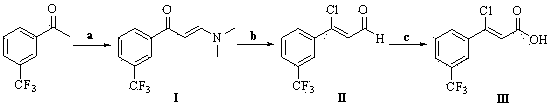
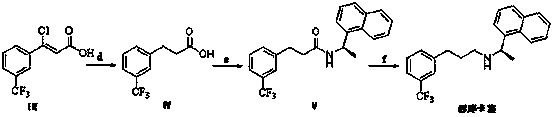
The invention relates to a preparation method of a cinacalcet intermediate (Z)-3-chlorine-3-[3-(trifluoromethyl)phenyl]-2-crylic acid. The preparation method comprises the following steps: condensing 3-(trifluoromethyl) acetophenone used as a starting material; reducing and performing other reactions to obtain the intermediate. The invention also relates to two methods for preparing cinacalcet by utilizing intermediate.
Owner:BEIJING WINSUNNY PHARMA CO LTD
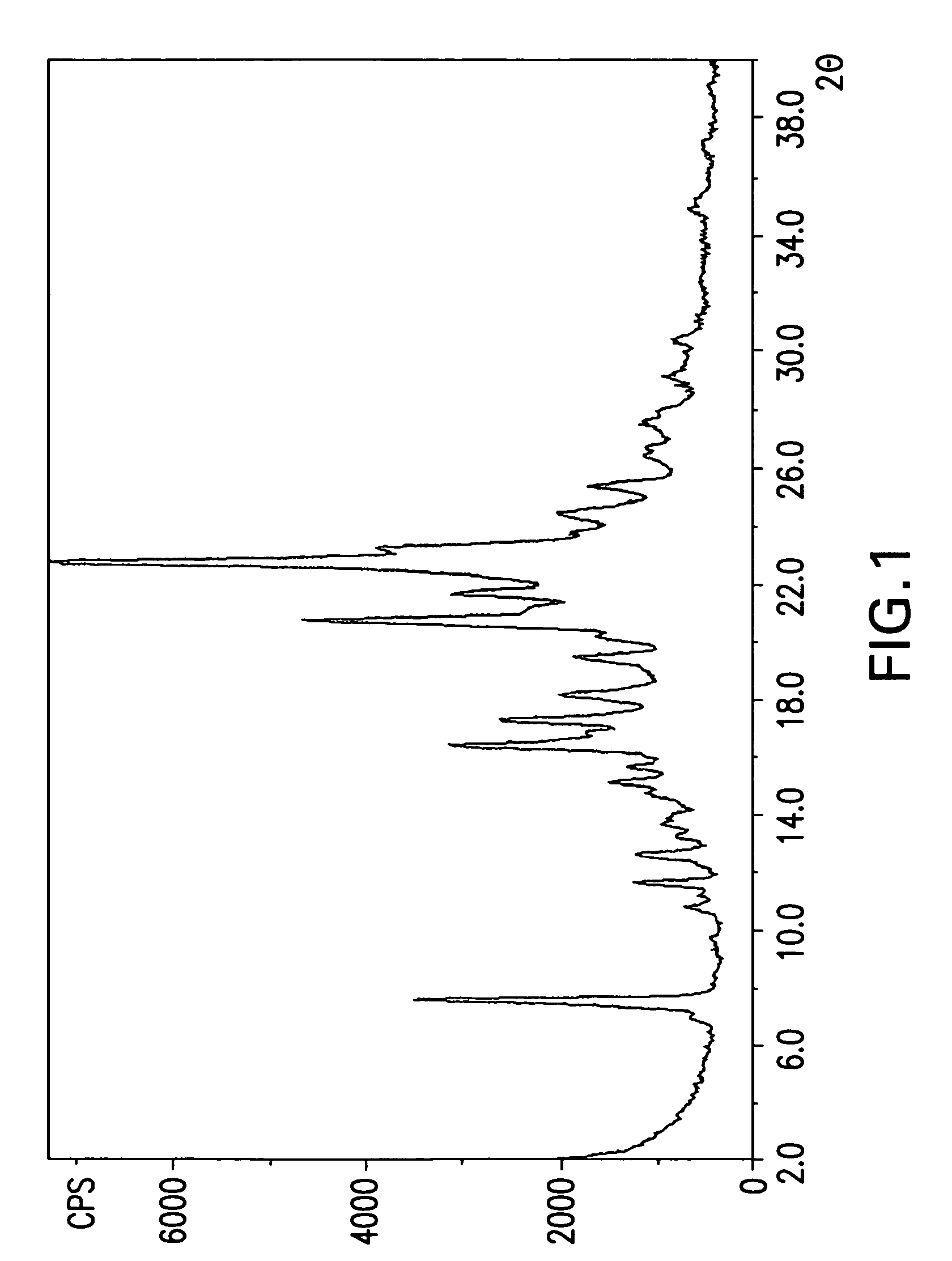
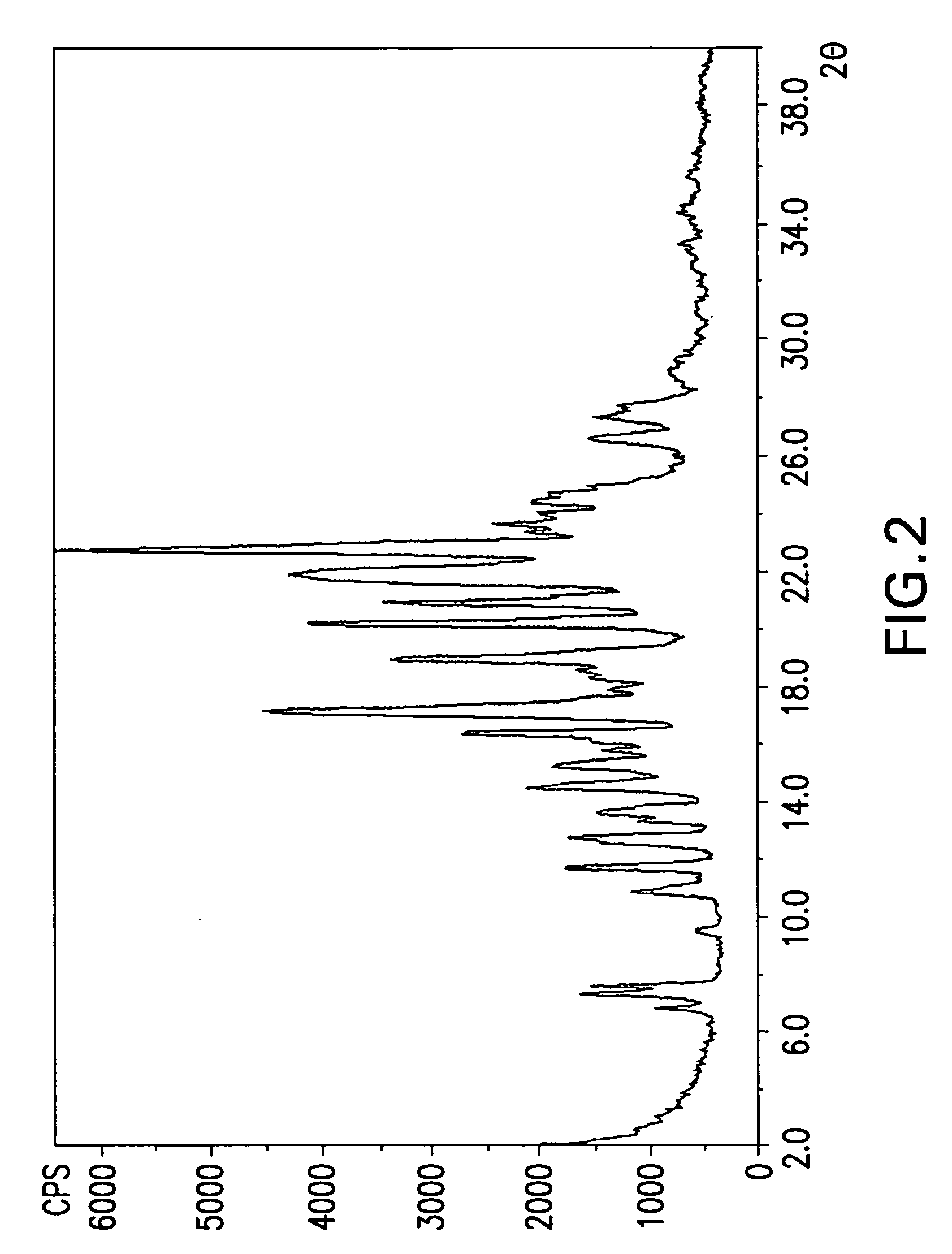
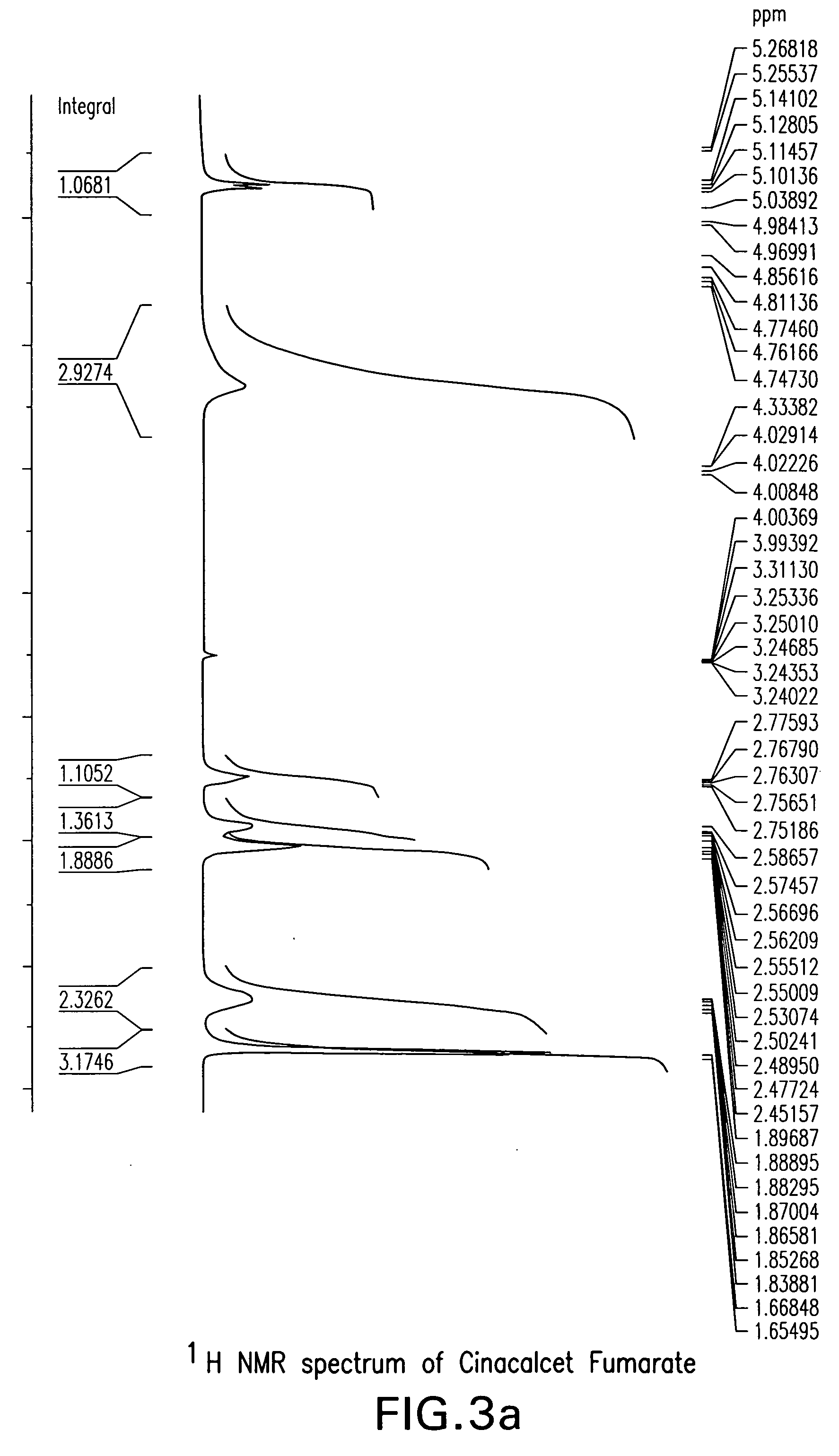
Crystalline forms cinacalcet fumarate and cinacalcet succinate and processes for preparation thereof
The present invention provides crystalline forms of Cinacalcet Fumarate and Cinacalcet Succinate, pharmaceutical compositions comprising the crystalline form of Cinacalcet Fumarate and / or the crystalline form of Cinacalcet Succinate, and processes for preparing the crystalline forms of Cinacalcet Fumarate and Cinacalcet Succinate and pharmaceutical compositions comprising the crystalline forms.
Owner:TEVA PHARM USA INC
Cinacalcet hydrochloride solid dispersion tablet and preparation technology thereof
InactiveCN105106144AHigh degreeSolve the preparation processOrganic active ingredientsPill deliveryMedicineCinacalcet Hydrochloride
The invention discloses a preparation technology of a Cinacalcet hydrochloride solid dispersion tablet, and belongs to the technical field of preparation of medicinal preparations. The technology is characterized in that the Cinacalcet hydrochloride solid dispersion tablet is prepared through a solid dispersion process and a powder direct tabletting process. The in vitro dissolution rate of Cinacalcet hydrochloride is characterized by 40-80% at 5-10min, 80-90% at 15-20min and 90-100% at 30-45min. The technology has the advantages of simple process, safety, controllable quality, low cost, short production cycle, and suitableness for large-scale industrial production.
Owner:SHENYANG PHARMA UNIVERSITY
Process for preparing cinacalcet and pharmaceutically acceptable salts thereof
InactiveUS20110172455A1Easy to useAmino compound purification/separationCarbamic acid derivatives preparationCarbamateNitrogen
The resent invention rovides a novel rocess for re arin cinacalcet of formula I and pharmaceutically acceptable salts thereof and process of purification. The present invention also provides novel nitrogen protected synthetic intermediates useful in the process of the present invention. Further, the present invention provides a novel substituted carbamate impurity and process of preparation thereof.
Owner:IND SWIFT LAB
Methods of synthesizing cinacalcet and salts thereof
InactiveUS20090137837A1Carbamic acid derivatives preparationOrganic compound preparationCombinatorial chemistryCinacalcet
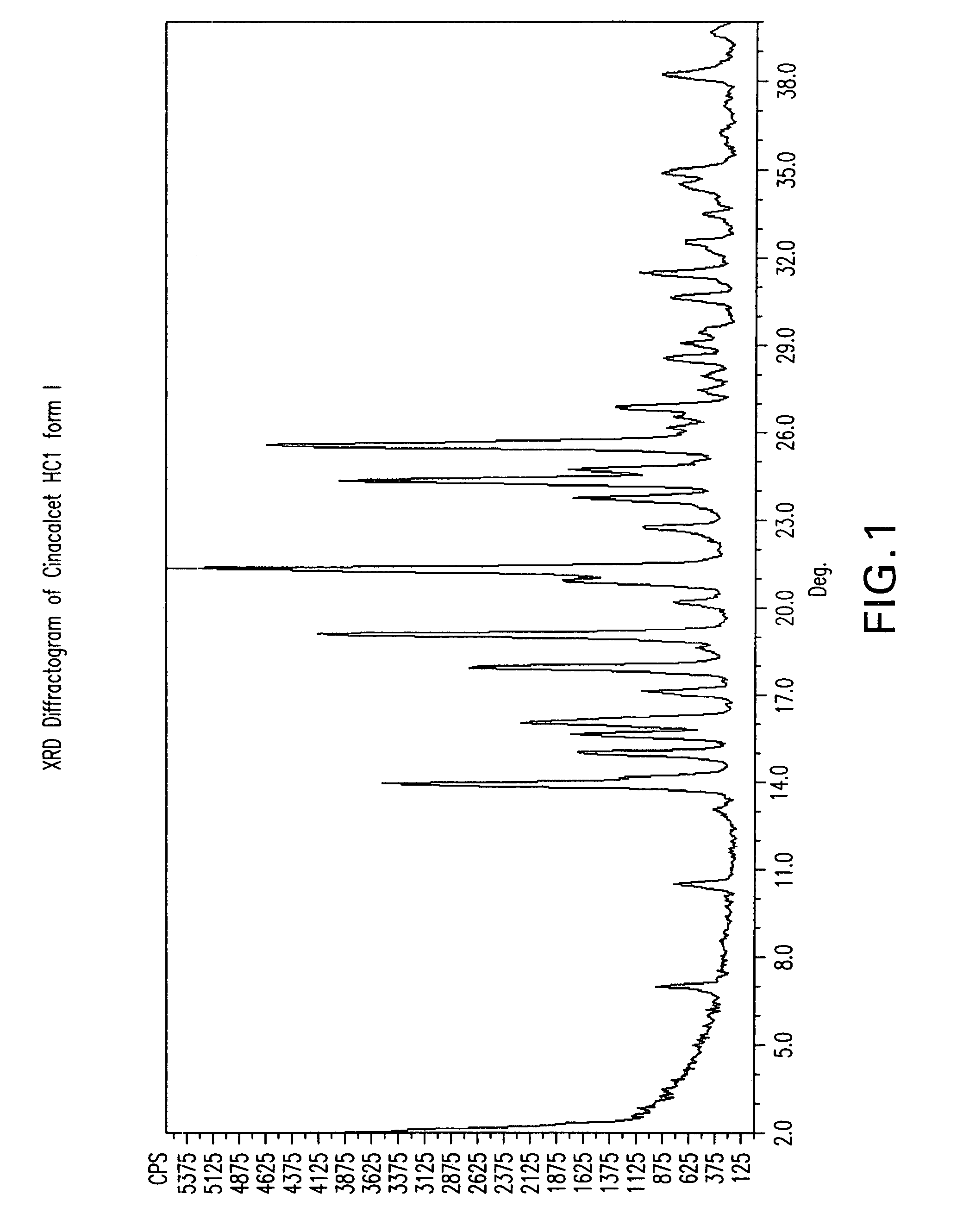
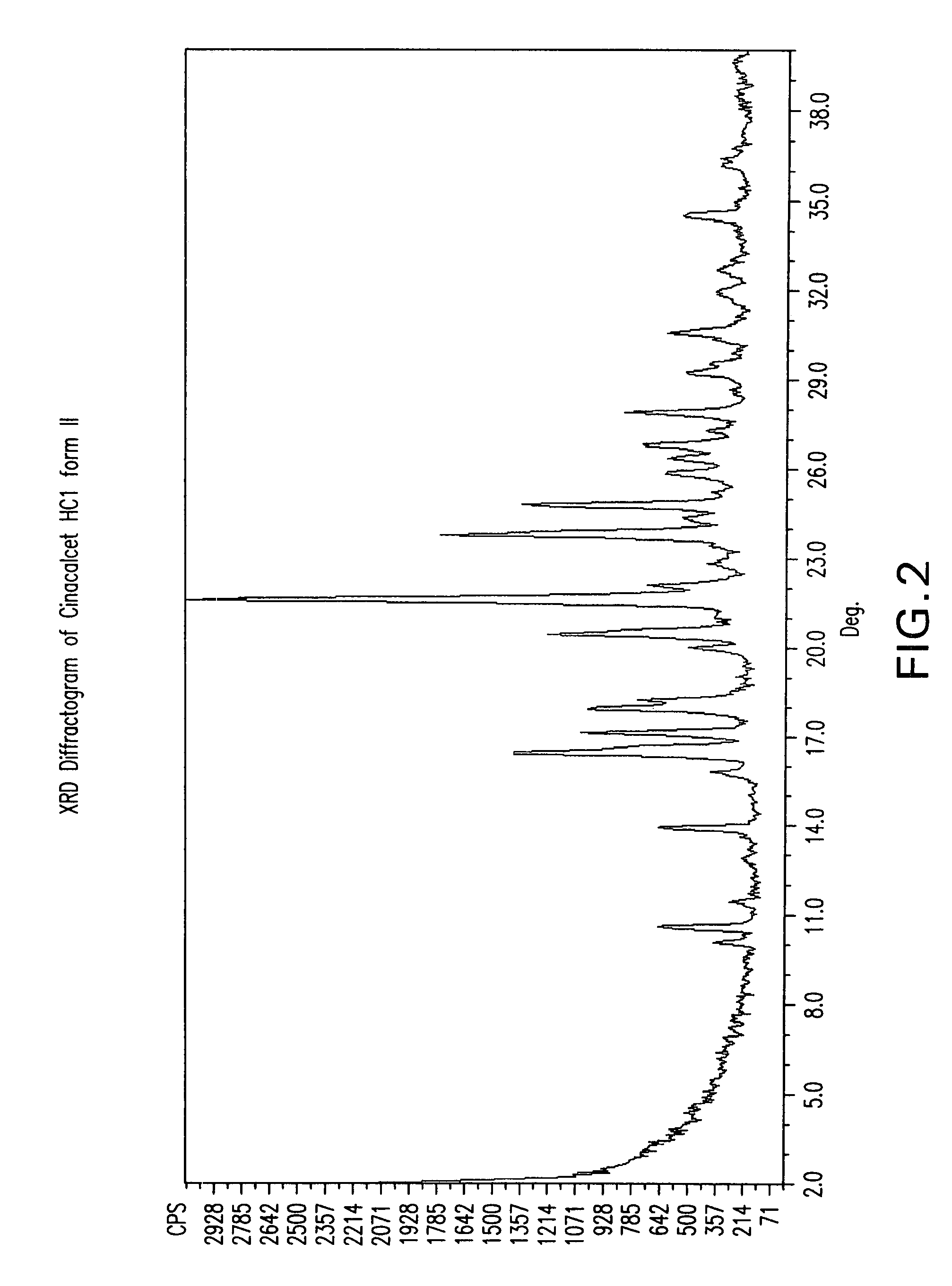
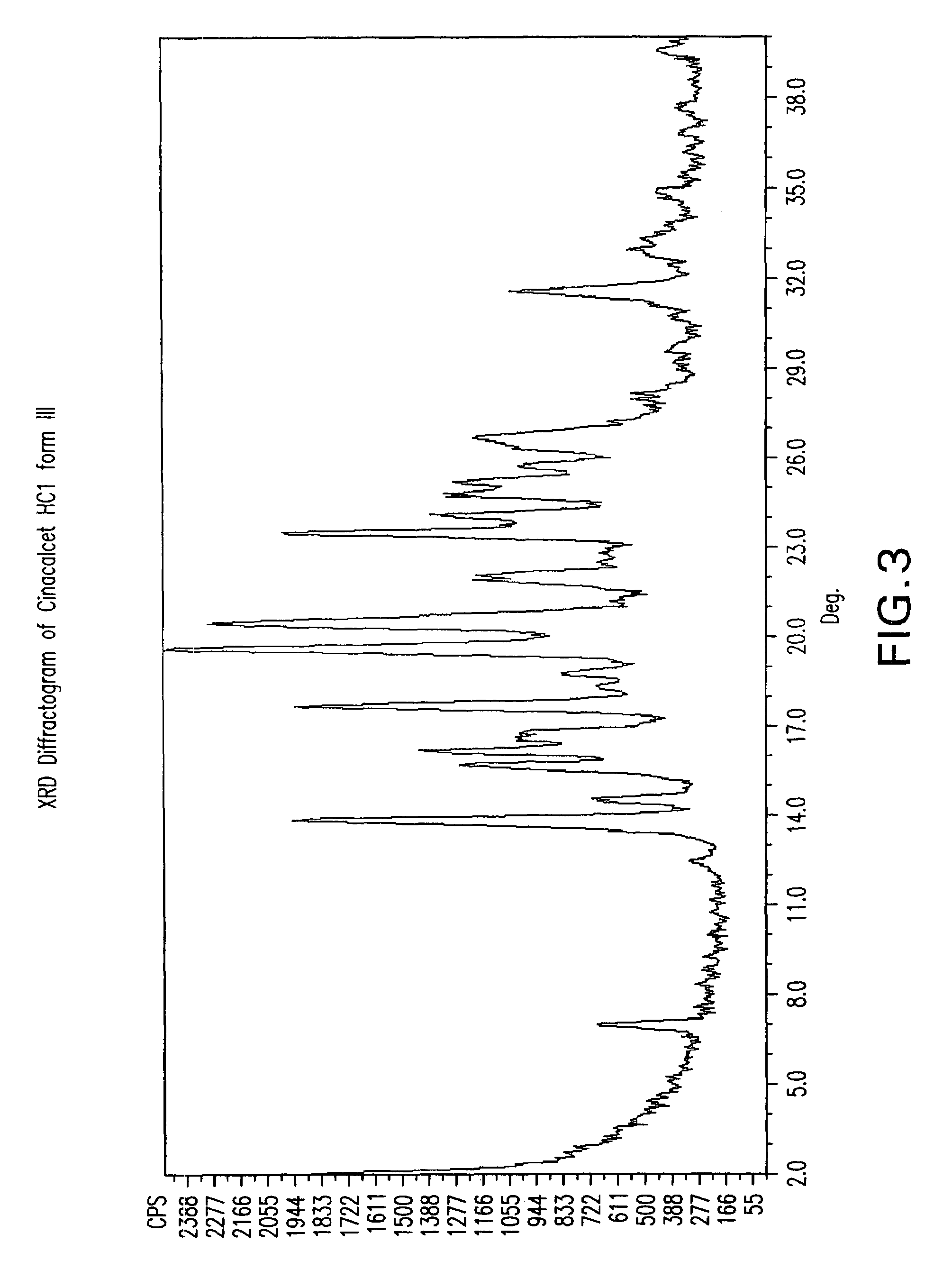
Methods of preparing cinacalcet, cinacalcet derivatives, and salts thereof is disclosed herein. Also disclosed herein are polymorphs of cinacalcet, compositions of cinacalcet, and methods of treating a subject by administering cinacalcet, wherein cinacalcet is prepared by the disclosed methods.
Owner:AMGEN INC
Therapeutic agents for regulating serum phosphorus
Methods for modulating serum phosphorus levels are described, wherein calcimimetic agents are administered to a subject in need thereof. In one embodiment, the compound is cinacalcet, and in other embodiments the compound is comprised of a contiguous sequence of subunits, X1—X2—X3—X4—X5—X6—X7, wherein the X1 subunit comprises a thiol-containing moiety and the distribution of charge on the X2-X7 subunits. The compound, when administered at selected times to a patient undergoing dialysis, lowers serum phosphorus levels, relative to pre-dosing levels, and achieves a sustained reduced level for a period of time after administration.
Owner:KAI PHARMA
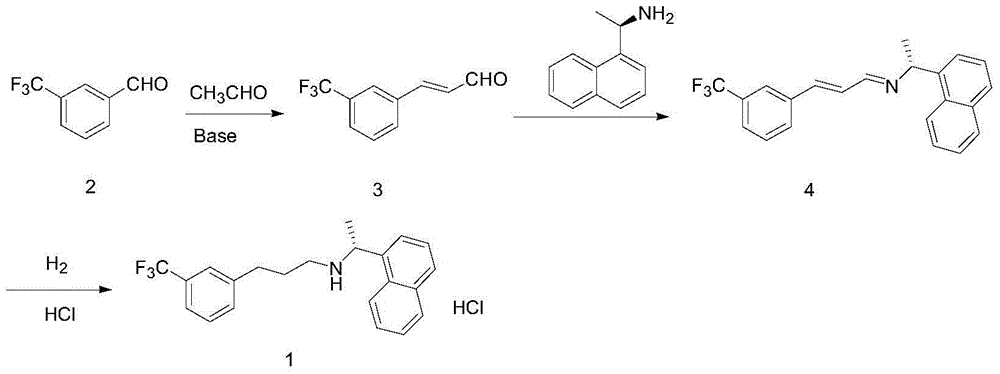
Synthesis method of cinacalcet
ActiveCN104592037AHigh yieldEasy to operateOrganic compound preparationAmino compound preparationP-ChlorophenolSynthesis methods
The invention discloses a synthesis method of cinacalcet. The synthetic route is divided into two parts, namely, (1) preparing a chiral compound as shown in the description from racemic 1-naphthylethylamine as a starting material by virtue of a dynamic kinetic reliquid method in the presence of Pd / LDH-SA serving as a racemic catalyst, p-chlorophenol fatty acyl ester serving as an acyl donor and lipase serving as a biological reliquid catalyst; and (2) reacting m-trifluoromethylbenzaldehyde serving as a starting material and a cheap and easily available material acetaldehyde to obtain m-trifluoromethyl cinnamic aldehyde and carrying out reduced pressure distillation to obtain a pure product; reacting m-trifluoromethyl cinnamic aldehyde and the compound as shown in the description to produce an imine intermediate; dissolving the imine intermediate in ethanol and reacting in the presence of Raney nickel serving as a hydrogenation catalyst to obtain the product cinacalcet. By the synthesis method, the reaction yield and the optical purity of the product are increased, the reaction conditions are milder and the raw materials are easily available.
Owner:ZHEJIANG UNIV
Method for synthesizing cinacalcet
InactiveCN104926665AAvoid residueThe synthesis method is simpleOrganic compound preparationAmino compound preparation by condensation/addition reactionsPropanoic acidMetal catalyst
The invention provides a method for synthesizing cinacalcet. According to the method, (R)-1-(1-naphthyl)ethylamine and 3-(3-trifluoromethylphenyl)propionic acid are adopted as raw materials; a nonmetal boron compound is adopted as a catalyst; an organosilane compound is adopted as a reducing agent; and a target product is synthesized with one step through a reductive coupling process. The method provided by the invention is simple and is easy to operate; and the raw materials are easy to obtain, such that cost is low. Also, no metal catalyst is needed in the synthesis process, such that metal residue in the drug is avoided, and the method is safe and environment-friendly. The method meets the requirements of green chemistry.
Owner:UNIV OF SCI & TECH OF CHINA
Preparation method of cinacalcet
InactiveCN103274948AReduce manufacturing costEasy to operateOrganic compound preparationAmino compound preparationCinacalcetSolvent
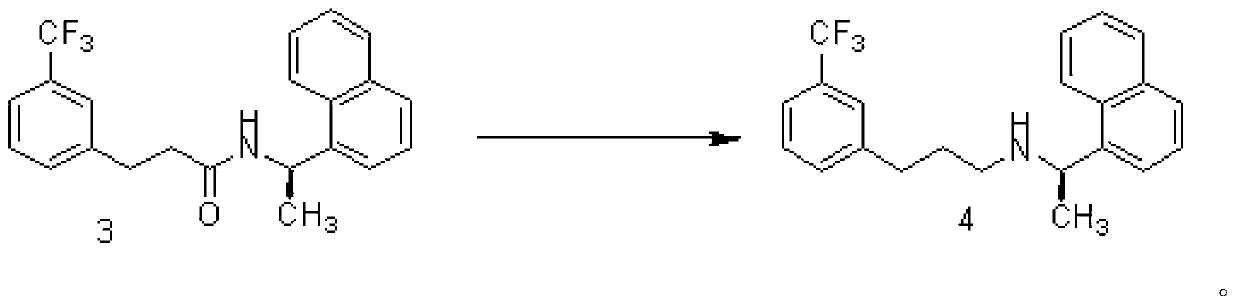
The invention provides a preparation method of cinacalcet, which comprises the following steps of: 1) mixing an alkali metal hydroboron, Lewis acid and a solvent (1); 2) mixing a compound shown in the formula (3) and a solvent (2), adding the mixture into a reaction product of the step 1), and partially steaming out the mixture until the internal temperature of the reaction liquid is 90-110 DEG C for reaction; and 3) collecting a compound shown in the formula (4) from the reaction product to obtain the target product: N-[1-(R)-(1-naphthyl ethyl)-N-[3-[3-(trifluoromethyl)phenyl]propyl]amine. Without a reagent with fairly great toxicity in the original document and through process improvement, the method provided by the invention has the advantages of low preparation cost, simplicity in operation and high yield, is suitable for industrial production and has substantial positive effects and application values. The reaction formula is shown in the specification.
Owner:SHANGHAI INST OF PHARMA IND +1
Method for preparing cinacalcet hydrochloride
ActiveCN106831441ALong synthetic stepsShort reaction stepsAmino preparation from aminesOrganic compound preparationPhenyl groupReducing agent
The invention discloses a method for preparing cinacalcet hydrochloride and belongs to the field of synthesis and preparation of chemical drugs. The method disclosed by the invention comprises the following steps: taking bromo-alpha,alpha,alpha-trifluorotoluene (II) and acryloyl chloride (III) as raw materials, and preparing m-trifluoromethyl acrylketone (IV) through a coupled reaction; carrying out an addition reaction between (IV) and (R)-1-(1-naphthyl) ethamine (V) so as to generate (R)-3-(1-(1-naphthyl)ethylamino)-1-(3-trifluoromethyl)phenyl)-1-acetone (VI); reducing (VI) to prepare N-((1R)-1-(1-naphthyl)ethyl)-3-(3-trifluoromethyl)phenyl)-1-propylamine (cinacalcet) (VII); carrying out a salt forming reaction on cinacalcet, thereby obtaining N-((1R)-1-(1-naphthyl)ethyl)-3-(3-trifluoromethyl)phenyl)-1-propylamine hydrochloride, namely the cinacalcet hydrochloride (I). According to the route, the cheap and readily available bromo-alpha,alpha,alpha-trifluorotoluene (II) and acryloyl chloride (III) are taken as the raw materials, a few steps are needed, usage of compounds of a heavy metal Pd and metal reducing agents LiAlH4, NaBH4 and the like is avoided, and the method is safe, environmentally friendly and economic and is suitable for large-scale industrial production.
Owner:JIANGSU SUZHONG PHARM GRP CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com